Ligand binding and hydration in protein misfolding: insights from studies of prion and p53 tumor suppressor proteins
- PMID: 19817406
- PMCID: PMC2825094
- DOI: 10.1021/ar900179t
Ligand binding and hydration in protein misfolding: insights from studies of prion and p53 tumor suppressor proteins
Abstract
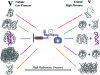
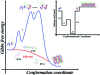
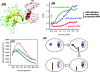
Protein misfolding has been implicated in a large number of diseases termed protein- folding disorders (PFDs), which include Alzheimer's disease, Parkinson's disease, transmissible spongiform encephalopathies, familial amyloid polyneuropathy, Huntington's disease, and type II diabetes. In these diseases, large quantities of incorrectly folded proteins undergo aggregation, destroying brain cells and other tissues. The interplay between ligand binding and hydration is an important component of the formation of misfolded protein species. Hydration drives various biological processes, including protein folding, ligand binding, macromolecular assembly, enzyme kinetics, and signal transduction. The changes in hydration and packing, both when proteins fold correctly or when folding goes wrong, leading to PFDs, are examined through several biochemical, biophysical, and structural approaches. Although in many cases the binding of a ligand such as a nucleic acid helps to prevent misfolding and aggregation, there are several examples in which ligands induce misfolding and assembly into amyloids. This occurs simply because the formation of structured aggregates (such as protofibrillar and fibrillar amyloids) involves decreases in hydration, formation of a hydrogen-bond network in the secondary structure, and burying of nonpolar amino acid residues, processes that also occur in the normal folding landscape. In this Account, we describe the present knowledge of the folding and misfolding of different proteins, with a detailed emphasis on mammalian prion protein (PrP) and tumoral suppressor protein p53; we also explore how ligand binding and hydration together influence the fate of the proteins. Anfinsen's paradigm that the structure of a protein is determined by its amino acid sequence is to some extent contradicted by the observation that there are two isoforms of the prion protein with the same sequence: the cellular and the misfolded isoform. The cellular isoform of PrP has a disordered N-terminal domain and a highly flexible, not-well-packed C-terminal domain, which might account for its significant hydration. When PrP binds to biological molecules, such as glycosaminoglycans and nucleic acids, the disordered segments appear to fold and become less hydrated. Formation of the PrP-nucleic acid complex seems to accelerate the conversion of the cellular form of the protein into the disease-causing isoform. For p53, binding to some ligands, including nucleic acids, would prevent misfolding of the protein. Recently, several groups have begun to analyze the folding-misfolding of the individual domains of p53, but several questions remain unanswered. We discuss the implications of these findings for understanding the productive and incorrect folding pathways of these proteins in normal physiological states and in human disease, such as prion disorders and cancer. These studies are shown to lay the groundwork for the development of new drugs.
Figures

References
-
- Schrödinger E.What Is Life? Mind and Matter; Cambridge University Press: 1944.
-
- Levy Y.; Onuchic J. N. Water Mediation in Protein Folding and Molecular Recognition. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 389–415. - PubMed
-
- Chiti F.; Dobson C. M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. - PubMed
-
- Foguel D.; Silva J. L. New Insights into the Mechanisms of Protein Misfolding and Aggregation in Amyloidogenic Diseases Derived From Pressure Studies. Biochemistry 2004, 43, 11361–11370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

