Mechanical signaling for bone modeling and remodeling
- PMID: 19817708
- PMCID: PMC3743123
- DOI: 10.1615/critreveukargeneexpr.v19.i4.50
Mechanical signaling for bone modeling and remodeling
Abstract
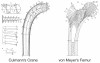
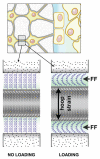
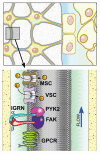
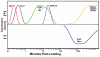
Proper development of the skeleton in utero and during growth requires mechanical stimulation. Loading results in adaptive changes in bone that strengthen bone structure. Bone's adaptive response is regulated by the ability of resident bone cells to perceive and translate mechanical energy into a cascade of structural and biochemical changes within the cells a process known as mechanotransduction. Mechanotransduction pathways are among the most anabolic in bone, and consequently, there is great interest in elucidating how mechanical loading produces its observed effects, including increased bone formation, reduced bone loss, changes in bone cell differentiation and lifespan, among others. A molecular understanding of these processes is developing, and with it comes a profound new insight into the biology of bone. In this article, we review the nature of the physical stimulus to which bone cells mount an adaptive response, including the identity of the sensor cells, their attributes and physical environment, and putative mechanoreceptors they express. Particular attention is allotted to the focal adhesion and Wnt signaling, in light of their emerging role in bone mechanotransduction. Te cellular mechanisms for increased bone loss during disuse, and reduced bone loss during loading are considered. Finally, we summarize the published data on bone cell accommodation, whereby bone cells stop responding to mechanical signaling events. Collectively, these data highlight the complex yet finely orchestrated process of mechanically regulated bone homeostasis.
Figures











References
-
- Wolff J. The law of bone transformation. A. Hirschwald; Berlin: 1892.
-
- Murray PDF . Bones – A study of the development and structure of the vertebrate skeleton. Cambridge University Press; Cambridge: 1936.
-
- von Meyer C. Die architektur der spongiosa. Arch Anat Physiol Wiss Med Reichert DuBois-Reymonds Arch. 1867;34:615–28.
-
- Rodriguez JI, Palacios J, Garcia-Alix A, Pastor I, Paniagua R. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int. 1988;43:335–9. - PubMed
-
- Ralis ZA, Ralis HM, Randall M, Watkins G, Blake PD. Changes in shape, ossification and quality of bones in children with spina bifida. Dev Med Child Neurol Suppl. 1976:29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

