Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis
- PMID: 19818015
- PMCID: PMC2802666
- DOI: 10.1111/j.1365-2958.2009.06910.x
Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis
Abstract
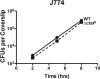
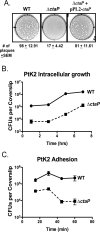
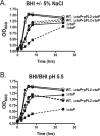
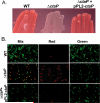
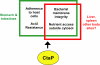
The bacterial pathogen Listeria monocytogenes survives under a myriad of conditions in the outside environment and within the human host where infections can result in severe disease. Bacterial life within the host requires the expression of genes with roles in nutrient acquisition as well as the biosynthesis of bacterial products required to support intracellular growth. A gene product identified as the substrate-binding component of a novel oligopeptide transport system (encoded by lmo0135) was recently shown to be required for L. monocytogenes virulence. Here we demonstrate that lmo0135 encodes a multifunctional protein that is associated with cysteine transport, acid resistance, bacterial membrane integrity and adherence to host cells. The lmo0135 gene product (designated CtaP, for cysteine transport associated protein) was required for bacterial growth in the presence of low concentrations of cysteine in vitro, but was not required for bacterial replication within the host cytosol. Loss of CtaP increased membrane permeability and acid sensitivity, and reduced bacterial adherence to host cells. ctaP deletion mutants were severely attenuated following intragastric and intravenous inoculation of mice. Taken together, the data presented indicate that CtaP contributes to multiple facets of L. monocytogenes physiology, growth and survival both inside and outside of animal cells.
Figures





References
-
- Alloing G, Martin B, Granadel C, Claverys JP. Development of competence in Streptococcus pneumonaie: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol Microbiol. 1998;29:75–83. - PubMed
-
- Berger EA, Heppel LA. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem. 1972;247:7684–7694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

