Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway
- PMID: 19818098
- PMCID: PMC3822730
- DOI: 10.1111/j.1582-4934.2009.00926.x
Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway
Abstract
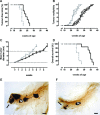
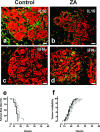
It is unknown whether zoledronic acid (ZA) at clinically relevant doses is active against tumours not located in bone. Mice transgenic for the activated ErbB-2 oncogene were treated with a cumulative number of doses equivalent to that recommended in human beings. A significant increase in tumour-free and overall survival was observed in mice treated with ZA. At clinically compatible concentrations, ZA modulated the mevalonate pathway and affected protein prenylation in both tumour cells and macrophages. A marked reduction in the number of tumour-associated macrophages was paralleled by a significant decrease in tumour vascularization. The local production of vascular endothelial growth factor and interleukin-10 was drastically down-regulated in favour of interferon-γ production. Peritoneal macrophages and tumour-associated macrophages of ZA-treated mice recovered a full M1 antitumoral phenotype, as shown by nuclear translocation of nuclear factor kB, inducible nitric oxide synthase expression and nitric oxide production. These data indicate that clinically achievable doses of ZA inhibit spontaneous mammary cancerogenesis by targeting the local microenvironment, as shown by a decreased tumour vascularization, a reduced number of tumour-associated macrophages and their reverted polarization from M2 to M1 phenotype.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



Similar articles
-
Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts.Oncotarget. 2017 Jan 3;8(1):118-132. doi: 10.18632/oncotarget.9497. Oncotarget. 2017. PMID: 27223431 Free PMC article.
-
Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma.Br J Cancer. 2010 Aug 24;103(5):629-41. doi: 10.1038/sj.bjc.6605814. Epub 2010 Jul 27. Br J Cancer. 2010. PMID: 20664588 Free PMC article.
-
Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models.Cell Oncol (Dordr). 2013 Dec;36(6):505-14. doi: 10.1007/s13402-013-0156-2. Epub 2013 Nov 1. Cell Oncol (Dordr). 2013. PMID: 24177992
-
Tumour macrophages as potential targets of bisphosphonates.J Transl Med. 2011 Oct 17;9:177. doi: 10.1186/1479-5876-9-177. J Transl Med. 2011. PMID: 22005011 Free PMC article. Review.
-
Direct antitumour activity of zoledronic acid: preclinical and clinical data.Clin Transl Oncol. 2011 Mar;13(3):148-55. doi: 10.1007/s12094-011-0634-9. Clin Transl Oncol. 2011. PMID: 21421459 Review.
Cited by
-
Correlation of the prognostic value of FNDC4 in glioblastoma with macrophage polarization.Cancer Cell Int. 2022 Sep 2;22(1):273. doi: 10.1186/s12935-022-02688-7. Cancer Cell Int. 2022. PMID: 36056336 Free PMC article.
-
Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth.Cancer Immunol Immunother. 2012 Sep;61(9):1373-85. doi: 10.1007/s00262-011-1178-0. Epub 2012 Jan 4. Cancer Immunol Immunother. 2012. PMID: 22215137 Free PMC article.
-
Dual identity of tumor-associated macrophage in regulated cell death and oncotherapy.Heliyon. 2023 Jun 26;9(7):e17582. doi: 10.1016/j.heliyon.2023.e17582. eCollection 2023 Jul. Heliyon. 2023. PMID: 37449180 Free PMC article. Review.
-
Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells.PLoS One. 2014 Jun 26;9(6):e101260. doi: 10.1371/journal.pone.0101260. eCollection 2014. PLoS One. 2014. PMID: 24968029 Free PMC article.
-
Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype.Sci Rep. 2017 Jan 3;7:39011. doi: 10.1038/srep39011. Sci Rep. 2017. PMID: 28045028 Free PMC article.
References
-
- Hillner BE, Ingle JN, Berenson JR, et al. American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. American Society of Clinical Oncology Bisphosphonates Expert Panel. J Clin Oncol. 2000;18:1378–91. - PubMed
-
- Roelofs AJ, Thompson K, Gordon S, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–30s. - PubMed
-
- Benford HL, Frith JC, Auriola S, et al. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol. 1999;56:131–40. - PubMed
-
- Benford HL, McGowan NW, Helfrich MH, et al. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–73. - PubMed
-
- Wolf AM, Rumpold H, Tilg H, et al. The effect of zoledronic acid on the function and differentiation of myeloid cells. Haematologica. 2006;91:1165–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

