Understanding the pharmacokinetics of Coartem
- PMID: 19818171
- PMCID: PMC2760239
- DOI: 10.1186/1475-2875-8-S1-S4
Understanding the pharmacokinetics of Coartem
Abstract
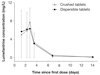
Artemether and lumefantrine (AL), the active constituents of Coartem exhibit complementary pharmacokinetic profiles. Artemether is absorbed quickly; peak concentrations of artemether and its main active metabolite, dihydroartemisinin (DHA) occur at approximately two hours post-dose, leading to a rapid reduction in asexual parasite mass and a prompt resolution of symptoms. Lumefantrine is absorbed and cleared more slowly (terminal elimination half-life 3-4 days in malaria patients), and accumulates with successive doses, acting to prevent recrudescence by destroying any residual parasites that remain after artemether and DHA have been cleared from the body. Food intake significantly enhances the bioavailability of both artemether and lumefantrine, an effect which is more apparent for the highly lipophilic lumefantrine. However, a meal with only a small amount of fat (1.6 g) is considered sufficient to achieve adequate exposure to lumefantrine. The pharmacokinetics of artemether or lumefantrine are similar in children, when dosed according to their body weight, compared with adults. No randomized study has compared the pharmacokinetics of either agent in pregnant versus non-pregnant women. Studies in healthy volunteers and in children with malaria have confirmed that the pharmacokinetic characteristics of crushed standard AL tablets and the newly-developed Coartem Dispersible tablet formulation are similar. Studies to date in healthy volunteers have not identified any clinically relevant drug-drug interactions; data relating to concomitant administration of HIV therapies are limited. While dose-response analyses are difficult to undertake because of the low rate of treatment failures under AL, it appears that artemether and DHA exposure impact on parasite clearance time while lumefantrine exposure is associated with cure rate, consistent with their respective modes of action. In conclusion, knowledge of the pharmacokinetic profiles of artemether and lumefantrine is increasing within a range of settings, including infants and children. However, additional data would be warranted to better characterize artemether and lumefantrine pharmacokinetics in patients with hepatic impairment, in pregnant women, and in patients undergoing HIV/AIDS chemotherapy.
Figures
Similar articles
-
Early clinical development of artemether-lumefantrine dispersible tablet: palatability of three flavours and bioavailability in healthy subjects.Malar J. 2010 Sep 3;9:253. doi: 10.1186/1475-2875-9-253. Malar J. 2010. PMID: 20815879 Free PMC article. Clinical Trial.
-
Evaluation of two novel tablet formulations of artemether-lumefantrine (Coartem) for bioequivalence in a randomized, open-label, two-period study.Malar J. 2013 Sep 8;12:312. doi: 10.1186/1475-2875-12-312. Malar J. 2013. PMID: 24010572 Free PMC article. Clinical Trial.
-
Increased systemic exposures of artemether and dihydroartemisinin in infants under 5 kg with uncomplicated Plasmodium falciparum malaria treated with artemether-lumefantrine (Coartem®).Malar J. 2015 Apr 15;14:157. doi: 10.1186/s12936-015-0682-7. Malar J. 2015. PMID: 25886021 Free PMC article. Clinical Trial.
-
Coartem: the journey to the clinic.Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S3. doi: 10.1186/1475-2875-8-S1-S3. Malar J. 2009. PMID: 19818170 Free PMC article. Review.
-
Coartemether (artemether and lumefantrine): an oral antimalarial drug.Expert Rev Anti Infect Ther. 2004 Apr;2(2):181-96. doi: 10.1586/14787210.2.2.181. Expert Rev Anti Infect Ther. 2004. PMID: 15482185 Review.
Cited by
-
CYP2B6*6 Genotype Specific Differences in Artemether-Lumefantrine Disposition in Healthy Volunteers.J Clin Pharmacol. 2020 Mar;60(3):351-360. doi: 10.1002/jcph.1527. Epub 2019 Sep 23. J Clin Pharmacol. 2020. PMID: 31549442 Free PMC article. Clinical Trial.
-
Artemether-Lumefantrine Combination Therapy for Treatment of Uncomplicated Malaria: The Potential for Complex Interactions with Antiretroviral Drugs in HIV-Infected Individuals.Malar Res Treat. 2011;2011:703730. doi: 10.4061/2011/703730. Epub 2011 Apr 6. Malar Res Treat. 2011. PMID: 22312573 Free PMC article.
-
Broadly reactive antibodies specific for Plasmodium falciparum MSP-1(19) are associated with the protection of naturally exposed children against infection.Malar J. 2012 Aug 21;11:287. doi: 10.1186/1475-2875-11-287. Malar J. 2012. PMID: 22909378 Free PMC article.
-
Assessment of the therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in northern KwaZulu-Natal: an observational cohort study.Malar J. 2012 Dec 28;11:434. doi: 10.1186/1475-2875-11-434. Malar J. 2012. PMID: 23272998 Free PMC article.
-
Differential Impact of Nevirapine on Artemether-Lumefantrine Pharmacokinetics in Individuals Stratified by CYP2B6 c.516G>T Genotypes.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e00947-19. doi: 10.1128/AAC.00947-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31871092 Free PMC article.
References
-
- Lefèvre G, Looareesuwan S, Treeprasertsuk S, Krudsood S, Silachamroon U, Gathmann I, Mull R, Bakshi R. A clinical and pharmacokinetic trial of six doses of artemether-lumefantrine for multidrug-resistant plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg. 2001;64:247–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources