Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis
- PMID: 19818451
- PMCID: PMC2784223
- DOI: 10.1016/j.jpeds.2009.07.049
Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis
Abstract
Objective: To determine long-term health benefits of nonablative bone marrow transplantation for severe combined immunodeficiency (SCID), we investigated our cohort of 161 related donor bone marrow-transplanted patients with SCID. Only 16 (10%) had HLA-identical donors.
Study design: All 124 survivors were sent questionnaires about their current clinical statuses. Details from clinic visits were also compiled. One hundred eleven patients (90%) were reached. We compared outcomes of patients transplanted before and after 3.5 months of life and by molecular defect.
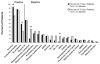
Results: The overall survival rate was 77%, but the rate for the 48 infants transplanted in the first 3.5 months of life was 94%, compared with 70% for the 113 transplanted after 3.5 months (P = .002). Twenty-eight (76%) of the 37 deceased patients died of viral infections present at diagnosis. One or more clinical problems were reported to have been present in the past 2 years in 71 (64%) of the survivors, although 95 (86%) were considered healthy by their families.
Conclusions: Most patients with SCID transplanted with related donor marrow without pretransplant chemotherapy have done well in the long term, but those transplanted at <3.5 months of age had a superior survival rate, a lower rate of clinical problems, less need for booster transplants, and better nutritional status.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. - PubMed
-
- Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. - PubMed
-
- Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136:2398–407. - PubMed
-
- Borghans JA, Bredius RG, Hazenberg MD, Roelofs H, Jol-van der Zijde EC, Heidt J, et al. Early determinants of long-term T-cell reconstitution after hematopoietic stem cell transplantation for severe combined immunodeficiency. Blood. 2006;108:763–9. - PubMed
-
- Honig M, Albert MH, Schulz A, Sparber-Sauer M, Schutz C, Belohradsky B, et al. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109:3595–602. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

