Lafora disease: insights into neurodegeneration from plant metabolism
- PMID: 19818631
- PMCID: PMC2805077
- DOI: 10.1016/j.tibs.2009.08.002
Lafora disease: insights into neurodegeneration from plant metabolism
Abstract
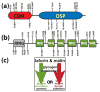
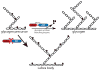
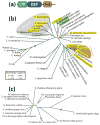
Reversible phosphorylation modulates nearly every step of glycogenesis and glycogenolysis. Multiple metabolic disorders are the result of defective enzymes that control these phosphorylation events, enzymes that were identified biochemically before the advent of the molecular biology era. Lafora disease is a metabolic disorder resulting in accumulation of water-insoluble glucan in the cytoplasm, and manifests as a debilitating neurodegeneration that ends with the death of the patient. Unlike most metabolic disorders, the link between Lafora disease and metabolism has not been defined in almost 100 years. The results of recent studies with mammalian cells, mouse models, eukaryotic algae, and plants have begun to define the molecular mechanisms that cause Lafora disease. The emerging theme identifies a new phosphorylation substrate in glycogen metabolism, the glucan itself.
Figures
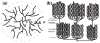


References
-
- Lafora G, Glick G. Beitrag zur histopathologie der myoklonischen epilepsie. Z Ges Neurol Psychiatr. 1911;6:1–14.
-
- Lafora GR. Uber des Vorkommen amyloider KJrperchen im innern der Ganglienzellen. Virchows Arch f Path Anat. 1911;205:295.
-
- Virchow RLK. Die Cellularpathologie inihrer Begründung auf physiologische and pathologische Gewebelehre. Berlin: Hirschwald; 1858.
-
- Yokoi S, Austin J, Witmer F. Isolation and characterization of Lafora bodies in two cases of myoclonus epilepsy. Journal of Neuropathology and Experimental Neurology. 1967;26(1):125–127. - PubMed
-
- Yokoi S, Austin J, Witmer F, Sakai M. Studies in myoclonus epilepsy (Lafora body form). I. Isolation and preliminary characterization of Lafora bodies in two cases. Arch Neurol. 1968;19(1):15–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

