How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets
- PMID: 19818653
- PMCID: PMC2998796
- DOI: 10.1016/j.immuni.2009.08.023
How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets
Abstract
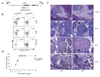
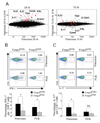
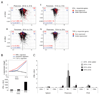
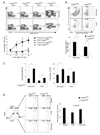
CD4(+)Foxp3(+) regulatory T cells (Treg cells) are known to control the progression of autoimmune diabetes, but when, where, and how they exert their influence in this context are questions still under vigorous debate. Exploiting a transgene encoding the human diphtheria toxin receptor, we punctually and specifically ablated Foxp3(+) cells in the BCD2.5/NOD mouse model of autoimmune diabetes. Strikingly, overt disease developed within 3 days. The earliest detectable event was the activation of natural killer (NK) cells directly within the insulitic lesion, particularly the induction of Ifng gene expression within 7 hours of Treg cell ablation. Interferon-gamma had a strong impact on the gene-expression program of the local CD4(+) T effector cell population, unleashing it to aggressively attack the islets, which was required for the development of diabetes. Thus, Treg cells regulate pancreatic autoimmunity in situ through control of a central innate immune system player, NK cells.
Figures


References
-
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:2021. - PubMed
-
- Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. - PubMed
-
- Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A. Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J. Immunol. 2001;166:2404–2411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

