Alpha4 is an essential regulator of PP2A phosphatase activity
- PMID: 19818709
- PMCID: PMC2761955
- DOI: 10.1016/j.molcel.2009.09.025
Alpha4 is an essential regulator of PP2A phosphatase activity
Abstract
The activity and specificity of serine/threonine phosphatases are governed largely by their associated proteins. alpha4 is an evolutionarily conserved noncatalytic subunit for PP2A-like phosphatases. Though alpha4 binds to only a minority of PP2A-related catalytic subunits, alpha4 deletion leads to progressive loss of all PP2A, PP4, and PP6 phosphatase complexes. In healthy cells, association with alpha4 renders catalytic (C) subunits enzymatically inactive while protecting them from proteasomal degradation until they are assembled into a functional phosphatase complex. During cellular stress, existing PP2A complexes can become unstable. Under such conditions, alpha4 sequesters released C subunits and is required for the adaptive increase in targeted PP2A activity that can dephosphorylate stress-induced phosphorylated substrates. Consistent with this, overexpression of alpha4 protects cells from a variety of stress stimuli, including DNA damage and nutrient limitation. These findings demonstrate that alpha4 plays a required role in regulating the assembly and maintenance of adaptive PP2A phosphatase complexes.
Figures

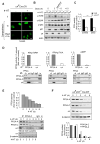

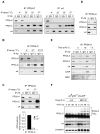
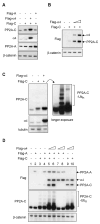

References
-
- Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. - PubMed
-
- Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J Biol Chem. 1998;273:19019–19024. - PubMed
-
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. - PubMed
-
- Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem Biophys Res Commun. 1998;247:827–832. - PubMed
-
- Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

