Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1
- PMID: 19819701
- PMCID: PMC4608440
- DOI: 10.1016/j.tibs.2009.07.009
Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1
Abstract
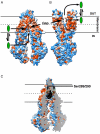
Multidrug ABC transporters can transport a wide range of drugs from the cell. Ongoing studies of the prototype mammalian multidrug resistance ATP-binding cassette transporter P-glycoprotein (ABCB1) have revealed many intriguing functional and biochemical features. However, a gap remains in our knowledge regarding the molecular basis of its broad specificity for structurally unrelated ligands. Recently, the first crystal structures of ligand-free and ligand-bound ABCB1 showed ligand binding in a cavity between its two membrane domains, and earlier observations on polyspecificity can now be interpreted in a structural context. Comparison of the new ABCB1 crystal structures with structures of bacterial homologs suggests a critical role for an axial rotation of transmembrane helices for high-affinity binding and low-affinity release of ligands during transmembrane transport.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures



References
-
- Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. - PubMed
-
- Borst P, Oude Elferink R. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002;71:537–592. - PubMed
-
- Gottesman MM, et al. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. - PubMed
-
- Holland IB, et al., editors. ABC Proteins: From Bacteria to Man. Academic Press; 2003.
-
- Verrier PJ, et al. Plant ABC proteins - a unified nomenclature and updated inventory. Trends Plant Sci. 2008;13:151–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

