Functional studies of split Arabidopsis Ca2+/H+ exchangers
- PMID: 19819871
- PMCID: PMC2797178
- DOI: 10.1074/jbc.M109.070235
Functional studies of split Arabidopsis Ca2+/H+ exchangers
Abstract
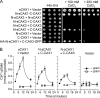
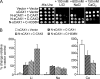
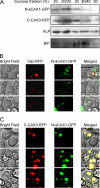
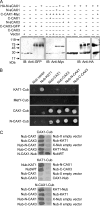
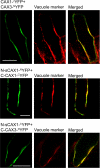
In plants, high capacity tonoplast cation/H(+) antiport is mediated in part by a family of cation exchanger (CAX) transporters. Functional association between CAX1 and CAX3 has previously been shown. In this study we further examine the interactions between CAX protein domains through the use of nonfunctional halves of CAX transporters. We demonstrate that a protein coding for an N-terminal half of an activated variant of CAX1 (sCAX1) can associate with the C-terminal half of either CAX1 or CAX3 to form a functional transporter that may exhibit unique transport properties. Using yeast split ubiquitin, in planta bimolecular fluorescence complementation, and gel shift experiments, we demonstrate a physical interaction among the half proteins. Moreover, the half-proteins both independently localized to the same yeast endomembrane. Co-expressing variants of N- and C-terminal halves of CAX1 and CAX3 in yeast suggested that the N-terminal region mediates Ca(2+) transport, whereas the C-terminal half defines salt tolerance phenotypes. Furthermore, in yeast assays, auto-inhibited CAX1 could be differentially activated by CAX split proteins. The N-terminal half of CAX1 when co-expressed with CAX1 activated Ca(2+) transport, whereas co-expressing C-terminal halves of CAX variants with CAX1 conferred salt tolerance but no apparent Ca(2+) transport. These findings demonstrate plasticity through hetero-CAX complex formation as well as a novel means to engineer CAX transport.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

