Docetaxel versus docetaxel alternating with gemcitabine as treatments of advanced breast cancer: final analysis of a randomised trial
- PMID: 19819914
- PMCID: PMC2860103
- DOI: 10.1093/annonc/mdp397
Docetaxel versus docetaxel alternating with gemcitabine as treatments of advanced breast cancer: final analysis of a randomised trial
Abstract
Background: Alternating administration of docetaxel and gemcitabine might result in improved time-to-treatment failure (TTF) and fewer adverse events compared with single-agent docetaxel as treatment of advanced breast cancer.
Patients and methods: Women diagnosed with advanced breast cancer were randomly allocated to receive 3-weekly docetaxel (group D) or 3-weekly docetaxel alternating with 3-weekly gemcitabine (group D/G) until treatment failure as first-line chemotherapy. The primary end point was TTF.
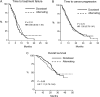
Results: Two hundred and thirty-seven subjects were assigned to treatment (group D, 115; group D/G, 122). The median TTF was 5.6 and 6.2 months in groups D and D/G, respectively (hazard ratio 0.85, 95% confidence interval 0.63-1.16; P = 0.31). There was no significant difference in time-to-disease progression, survival, and response rate between the groups. When adverse events were evaluated for the worst toxicity encountered during treatment, there was little difference between the groups, but when they were assessed per cycle, alternating treatment was associated with fewer severe (grade 3 or 4) adverse effects (P = 0.013), and the difference was highly significant for cycles when gemcitabine was administered in group D/G (P < 0.001).
Conclusion: The alternating regimen was associated with a similar TTF as single-agent docetaxel but with fewer adverse effects during gemcitabine cycles.
Figures
References
-
- Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. - PubMed
-
- Chan S, Friedrichs K, Noel D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17:2341–2354. - PubMed
-
- Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol. 2000;18:724–733. - PubMed
-
- Montero A, Fossella F, Hortobagyi G, et al. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–239. - PubMed
-
- Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous