Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis
- PMID: 19819944
- PMCID: PMC2775979
- DOI: 10.1210/en.2009-0532
Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis
Abstract
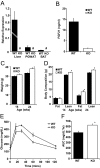
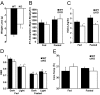
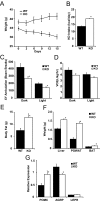
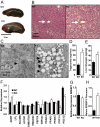
Fibroblast growth factor 21 (FGF21) is a key metabolic regulator. Expressed primarily in liver and adipose tissue, FGF21 is induced via peroxisome proliferator-activated receptor (PPAR) pathways during states requiring increased fatty acid oxidation including fasting and consumption of a ketogenic diet. To test the hypothesis that FGF21 is a physiological regulator that plays a role in lipid oxidation, we generated mice with targeted disruption of the Fgf21 locus (FGF21 knockout). Mice lacking FGF21 had mild weight gain and slightly impaired glucose homeostasis, indicating a role in long-term energy homeostasis. Furthermore, FGF21KO mice tolerated a 24-h fast, indicating that FGF21 is not essential in the early stages of starvation. In contrast to wild-type animals in which feeding KD leads to dramatic weight loss, FGF21KO mice fed KD gained weight, developed hepatosteatosis, and showed marked impairments in ketogenesis and glucose control. This confirms the physiological importance of FGF21 in the adaptation to KD feeding. At a molecular level, these effects were accompanied by lower levels of expression of PGC1alpha and PGC1beta in FGF21KO mice, strongly implicating these key transcriptional regulators in the action of FGF21. Furthermore, within the liver, the maturation of the lipogenic transcription factor sterol regulatory element-binding protein-1c was increased in FGF21KO mice, implicating posttranscriptional events in the maladaptation of FGF21KO mice to KD. These data reinforce the role of FGF21 is a critical regulator of long-term energy balance and metabolism. Mice lacking FGF21 cannot respond appropriately to a ketogenic diet, resulting in an impaired ability to mobilize and utilize lipids.
Figures
References
-
- Moore DD 2007 Physiology. Sister act. Science 316:1436–1438 - PubMed
-
- Kurosu H, Kuro-O M 2009 The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol 299:72–78 - PubMed
-
- Nishimura T, Nakatake Y, Konishi M, Itoh N 2000 Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492:203–206 - PubMed
-
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB 2005 FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 - PMC - PubMed
-
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ 2007 The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

