Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice
- PMID: 19819967
- PMCID: PMC2775990
- DOI: 10.1210/en.2009-0500
Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice
Abstract
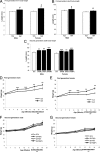
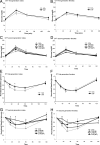
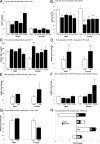
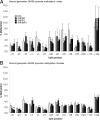
Maternal obesity and diet consumption during pregnancy have been linked to offspring adiposity, cardiovascular disease, and impaired glucose metabolism. Furthermore, nutrition during development is clearly linked to somatic growth. However, few studies have examined whether phenotypes derived from maternal high-fat diet exposure can be passed to subsequent generations and by what mechanisms this may occur. Here we report the novel finding of a significant body length increase that persisted across at least two generations of offspring in response to maternal high-fat diet exposure. This phenotype is not attributable to altered intrauterine conditions or maternal feeding behavior because maternal and paternal lineages were able to transmit the effect, supporting a true epigenetic manner of inheritance. We also detected a heritable feature of reduced insulin sensitivity across two generations. Alterations in the GH secretagogue receptor (GHSR), the GHSR transcriptional repressor AF5q31, plasma IGF-I concentrations, and IGF-binding protein-3 (IGFBP3) suggest a contribution of the GH axis. These studies provide evidence that the heritability of body length and glucose homeostasis are modulated by maternal diet across multiple generations, providing a mechanism where length can increase rapidly in concert with caloric availability.
Figures

References
-
- James WP 2008 The epidemiology of obesity: the size of the problem. J Intern Med 263:336–352 - PubMed
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 - PubMed
-
- Ni Mhurchu C, Rodgers A, Pan WH, Gu DF, Woodward M; Asia Pacific Cohort Studies Collaboration 2004 Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol 33:751–758 - PubMed
-
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF 2006 Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355:763–778 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

