The impact of water deficiency on leaf cuticle lipids of Arabidopsis
- PMID: 19819982
- PMCID: PMC2785987
- DOI: 10.1104/pp.109.141911
The impact of water deficiency on leaf cuticle lipids of Arabidopsis
Abstract
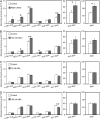
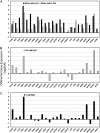
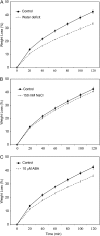
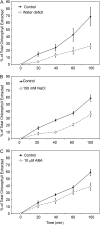
Arabidopsis (Arabidopsis thaliana) plants subjected to water deficit, sodium chloride (NaCl), or abscisic acid treatments were shown to exhibit a significant increase in the amount of leaf cuticular lipids. These stress treatments led to increases in cuticular wax amount per unit area of 32% to 80%, due primarily to 29% to 98% increases in wax alkanes. Of these treatments, only water deficit increased the total cutin monomer amount (by 65%), whereas both water deficit and NaCl altered the proportional amounts of cutin monomers. Abscisic acid had little effect on cutin composition. Water deficit, but not NaCl, increased leaf cuticle thickness (by 49%). Electron micrographs revealed that both water-deprived and NaCl-treated plants had elevated osmium accumulation in their cuticles. The abundance of cuticle-associated gene transcripts in leaves was altered by all treatments, including those performed in both pot-grown and in vitro conditions. Notably, the abundance of the ECERIFERUM1 gene transcript, predicted to function in alkane synthesis, was highly induced by all treatments, results consistent with the elevated alkane amounts observed in all treatments. Further, this induction of cuticle lipids was associated with reduced cuticle permeability and may be important for plant acclimation to subsequent water-limited conditions. Taken together, these results show that Arabidopsis provides an excellent model system to study the role of the cuticle in plant response to drought and related stresses, and its associated genetic and cellular regulation.
Figures


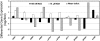

References
-
- Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15 413–428
-
- Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24 23–58
-
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6,cis-9-diene-1,18-dioate as the major component. Plant J 40 920–930 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

