Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet {alpha}-cells but not of intestinal L-cells
- PMID: 19819987
- PMCID: PMC5419124
- DOI: 10.1210/me.2009-0296
Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet {alpha}-cells but not of intestinal L-cells
Abstract
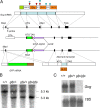
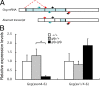
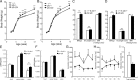
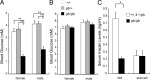
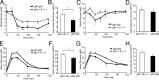
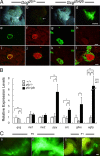
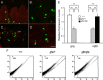
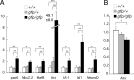
Multiple bioactive peptides, including glucagon, glucagon-like peptide-1 (GLP-1), and GLP-2, are derived from the glucagon gene (Gcg). In the present study, we disrupted Gcg by introduction of GFP cDNA and established a knock-in mouse line. Gcg(gfp/gfp) mice that lack most, if not all, of Gcg-derived peptides were born in an expected Mendelian ratio without gross abnormalities. Gcg(gfp/gfp) mice showed lower blood glucose levels at 2 wk of age, but those in adult Gcg(gfp/gfp) mice were not significantly different from those in Gcg(+/+) and Gcg(gfp/+) mice, even after starvation for 16 h. Serum insulin levels in Gcg(gfp/gfp) mice were lower than in Gcg(+/+) and Gcg(gfp/+) on ad libitum feeding, but no significant differences were observed on starvation. Islet alpha-cells and intestinal L-cells were readily visualized in Gcg(gfp/gfp) and Gcg(gfp/+) mice under fluorescence. The Gcg(gfp/gfp) postnatally developed hyperplasia of islet alpha-cells, whereas the population of intestinal L-cells was not increased. In the Gcg(gfp/gfp), expression of Aristaless-related homeobox (Arx) was markedly increased in pancreas but not in intestine and suggested involvement of Arx in differential regulation of proliferation of Gcg-expressing cells. These results illustrated that Gcg-derived peptides are dispensable for survival and maintaining normoglycemia in adult mice and that Gcg-derived peptides differentially regulate proliferation/differentiation of alpha-cells and L-cells. The present model is useful for analyzing glucose/energy metabolism in the absence of Gcg-derived peptides. It is useful also for analysis of the development, differentiation, and function of Gcg-expressing cells, because such cells are readily visualized by fluorescence in this model.
Figures
Similar articles
-
Aristaless-related homeobox plays a key role in hyperplasia of the pancreas islet α-like cells in mice deficient in proglucagon-derived peptides.PLoS One. 2013 May 9;8(5):e64415. doi: 10.1371/journal.pone.0064415. Print 2013. PLoS One. 2013. PMID: 23671715 Free PMC article.
-
Metabolic impact of glucagon deficiency.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:151-7. doi: 10.1111/j.1463-1326.2011.01456.x. Diabetes Obes Metab. 2011. PMID: 21824269 Review.
-
Fertility and pregnancy-associated ß-cell proliferation in mice deficient in proglucagon-derived peptides.PLoS One. 2012;7(8):e43745. doi: 10.1371/journal.pone.0043745. Epub 2012 Aug 23. PLoS One. 2012. PMID: 22928026 Free PMC article.
-
High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting.Mol Metab. 2017 Jan 12;6(3):236-244. doi: 10.1016/j.molmet.2017.01.003. eCollection 2017 Mar. Mol Metab. 2017. PMID: 28271030 Free PMC article.
-
Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease.Physiol Rev. 2015 Apr;95(2):513-48. doi: 10.1152/physrev.00013.2014. Physiol Rev. 2015. PMID: 25834231 Review.
Cited by
-
Deconstructing pancreas developmental biology.Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a012401. doi: 10.1101/cshperspect.a012401. Cold Spring Harb Perspect Biol. 2012. PMID: 22587935 Free PMC article. Review.
-
Endogenous GIP ameliorates impairment of insulin secretion in proglucagon-deficient mice under moderate beta cell damage induced by streptozotocin.Diabetologia. 2016 Jul;59(7):1533-1541. doi: 10.1007/s00125-016-3935-2. Epub 2016 Apr 6. Diabetologia. 2016. PMID: 27053237 Free PMC article.
-
Pancreatic islet-autonomous insulin and smoothened-mediated signalling modulate identity changes of glucagon+ α-cells.Nat Cell Biol. 2018 Nov;20(11):1267-1277. doi: 10.1038/s41556-018-0216-y. Epub 2018 Oct 22. Nat Cell Biol. 2018. PMID: 30361701 Free PMC article.
-
Novel time-resolved reporter mouse reveals spatial and transcriptional heterogeneity during alpha cell differentiation.Diabetologia. 2024 Jan;67(1):156-169. doi: 10.1007/s00125-023-06028-w. Epub 2023 Oct 23. Diabetologia. 2024. PMID: 37870650
-
SEL1L-HRD1 ER-Associated Degradation Facilitates Prohormone Convertase 2 Maturation and Glucagon Production in Islet α Cells.bioRxiv [Preprint]. 2025 Mar 20:2025.03.20.644437. doi: 10.1101/2025.03.20.644437. bioRxiv. 2025. PMID: 40166183 Free PMC article. Preprint.
References
-
- Kieffer TJ, Habener JF1999. The glucagon-like peptides. Endocr Rev 20:876–913 - PubMed
-
- Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, Carroll R, Steiner DF2005. Significance of prohormone convertase 2, PC2, mediated initial cleavage at the proglucagon interdomain site, Lys70-Arg71, to generate glucagon. Endocrinology 146:713–727 - PubMed
-
- Drucker DJ2006. The biology of incretin hormones. Cell Metab 3:153–165 - PubMed
-
- Lovshin JA, Drucker DJ2009. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 - PubMed
-
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ2003. Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100:1438–1443 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

