Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin
- PMID: 19820086
- PMCID: PMC2805316
- DOI: 10.1128/JB.01028-09
Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin
Abstract
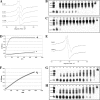
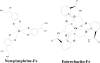
The ability of catecholamine stress hormones and inotropes to stimulate the growth of infectious bacteria is now well established. A major element of the growth induction process has been shown to involve the catecholamines binding to the high-affinity ferric-iron-binding proteins transferrin (Tf) and lactoferrin, which then enables bacterial acquisition of normally inaccessible sequestered host iron. The nature of the mechanism(s) by which the stress hormones perturb iron binding of these key innate immune defense proteins has not been fully elucidated. The present study employed electron paramagnetic resonance spectroscopy and chemical iron-binding analyses to demonstrate that catecholamine stress hormones form direct complexes with the ferric iron within transferrin and lactoferrin. Moreover, these complexes were shown to result in the reduction of Fe(III) to Fe(II) and the loss of protein-complexed iron. The use of bacterial ferric iron uptake mutants further showed that both the Fe(II) and Fe(III) released from the Tf could be directly used as bacterial nutrient sources. We also analyzed the transferrin-catecholamine interactions in human serum and found that therapeutically relevant concentrations of stress hormones and inotropes could directly affect the iron binding of serum-transferrin so that the normally highly bacteriostatic tissue fluid became significantly more supportive of the growth of bacteria. The relevance of these catecholamine-transferrin/lactoferrin interactions to the infectious disease process is considered.
Figures

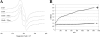

References
-
- Bailey, M., H. Engler, and J. Sheridan. 2006. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J. Neuroimmunol. 171:29-37. - PubMed
-
- Borisenko, G., A. Kagan, C. Hsia, and N. F. Schor. 2000. Interaction between 6-hydroxydopamine and transferrin: “Let My Iron Go.” Biochemistry 39:3392-3400. - PubMed
-
- Burton, C. L., S. R. Chhabra, S. Swift, T. J. Baldwin, H. Withers, S. J. Hill, and P. Williams. 2002. The growth response of Escherichia coli to neurotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect. Immun. 70:5913-5923. - PMC - PubMed
-
- El-Ayaan, U., R. F. Jameson, and W. Linert. 1998. A kinetic study of the reaction between noradrenaline and iron(III): an example of parallel inner- and outer-sphere electron transfer. J. Chem. Soc. Dalton Trans. 1998:1315-1319.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

