Engineering anatomically shaped human bone grafts
- PMID: 19820164
- PMCID: PMC2840502
- DOI: 10.1073/pnas.0905439106
Engineering anatomically shaped human bone grafts
Abstract
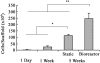
The ability to engineer anatomically correct pieces of viable and functional human bone would have tremendous potential for bone reconstructions after congenital defects, cancer resections, and trauma. We report that clinically sized, anatomically shaped, viable human bone grafts can be engineered by using human mesenchymal stem cells (hMSCs) and a "biomimetic" scaffold-bioreactor system. We selected the temporomandibular joint (TMJ) condylar bone as our tissue model, because of its clinical importance and the challenges associated with its complex shape. Anatomically shaped scaffolds were generated from fully decellularized trabecular bone by using digitized clinical images, seeded with hMSCs, and cultured with interstitial flow of culture medium. A bioreactor with a chamber in the exact shape of a human TMJ was designed for controllable perfusion throughout the engineered construct. By 5 weeks of cultivation, tissue growth was evidenced by the formation of confluent layers of lamellar bone (by scanning electron microscopy), markedly increased volume of mineralized matrix (by quantitative microcomputer tomography), and the formation of osteoids (histologically). Within bone grafts of this size and complexity cells were fully viable at a physiologic density, likely an important factor of graft function. Moreover, the density and architecture of bone matrix correlated with the intensity and pattern of the interstitial flow, as determined in experimental and modeling studies. This approach has potential to overcome a critical hurdle-in vitro cultivation of viable bone grafts of complex geometries-to provide patient-specific bone grafts for craniofacial and orthopedic reconstructions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Feinberg SE, Hollister SJ, Halloran JW, Chu TMG, Krebsbach PH. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. - PubMed
-
- Alhadlaq A, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. - PubMed
-
- Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951–956. - PubMed
-
- Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surgery Am. 2005;87:936–944. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

