Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination
- PMID: 19820184
- PMCID: PMC2761112
- DOI: 10.1242/dev.042242
Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination
Abstract
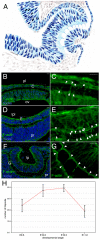
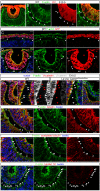
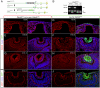
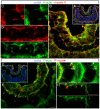
The vertebrate lens provides an excellent model with which to study the mechanisms required for epithelial invagination. In the mouse, the lens forms from the head surface ectoderm. A domain of ectoderm first thickens to form the lens placode and then invaginates to form the lens pit. The epithelium of the lens placode remains in close apposition to the epithelium of the presumptive retina as these structures undergo a coordinated invagination. Here, we show that F-actin-rich basal filopodia that link adjacent presumptive lens and retinal epithelia function as physical tethers that coordinate invagination. The filopodia, most of which originate in the presumptive lens, form at E9.5 when presumptive lens and retinal epithelia first come into close contact, and have retracted by E11.5 when invagination is complete. At E10.5--the lens pit stage--there is approximately one filopodium per epithelial cell. Formation of filopodia is dependent on the Rho family GTPase Cdc42 and the Cdc42 effector IRSp53 (Baiap2). Loss of filopodia results in reduced lens pit invagination. Pharmacological manipulation of the actin-myosin contraction pathway showed that the filopodia can respond rapidly in length to change inter-epithelial distance. These data suggest that the lens-retina inter-epithelial filopodia are a fine-tuning mechanism to assist in lens pit invagination by transmitting the forces between presumptive lens and retina. Although invagination of the archenteron in sea urchins and dorsal closure in Drosophila are known to be partly dependent on filopodia, this mechanism of morphogenesis has not previously been identified in vertebrates.
Figures


References
-
- Banzai, Y., Miki, H., Yamaguchi, H. and Takenawa, T. (2000). Essential role of neural Wiskott-Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J. Biol. Chem. 275, 11987-11992. - PubMed
-
- Beggs, H. E., Schahin-Reed, D., Zang, K., Goebbels, S., Nave, K. A., Gorski, J., Jones, K. R., Sretavan, D. and Reichardt, L. F. (2003). FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40, 501-514. - PMC - PubMed
-
- Boussadia, O., Kutsch, S., Hierholzer, A., Delmas, V. and Kemler, R. (2002). E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115, 53-62. - PubMed
-
- Brahmbhatt, A. A. and Klemke, R. L. (2003). ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J. Biol. Chem. 278, 13016-13025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

