Photoactivable sphingosine as a tool to study membrane microenvironments in cultured cells
- PMID: 19820263
- PMCID: PMC2842147
- DOI: 10.1194/jlr.M001974
Photoactivable sphingosine as a tool to study membrane microenvironments in cultured cells
Abstract
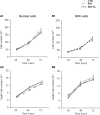
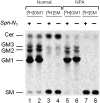
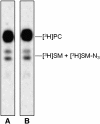
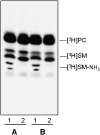
Human fibroblasts from normal subjects and Niemann-Pick A (NPA) disease patients were fed with two labeled metabolic precursors of sphingomyelin (SM), [(3)H]choline and photoactivable sphingosine, that entered into the biosynthetic pathway allowing the synthesis of radioactive phosphatidylcholine and SM, and of radioactive and photoactivable SM ([(3)H]SM-N(3)). Detergent resistant membrane (DRM) fractions prepared from normal and NPA fibroblasts resulted as highly enriched in [(3)H]SM-N(3). However, lipid and protein analysis showed strong differences between the two cell types. After cross-linking, different patterns of SM-protein complexes were found, mainly associated with the detergent soluble fraction of the gradient containing most cell proteins. After cell surface biotinylation, DRMs were immunoprecipitated using streptavidin. In conditions that maintain the integrity of domain, SM-protein complexes were detectable only in normal fibroblasts, whereas disrupting the membrane organization, these complexes were not recovered in the immunoprecipitate, suggesting that they involve proteins belonging to the inner membrane layer. These data suggest that differences in lipid and protein compositions of these cell lines determine specific lipid-protein interactions and different clustering within plasma membrane. In addition, our experiments show that photoactivable sphingolipids metabolically synthesized in cells can be used to study sphingolipid protein environments and sphingolipid-protein interactions.
Figures






Similar articles
-
Immunoseparation of sphingolipid-enriched membrane domains enriched in Src family protein tyrosine kinases and in the neuronal adhesion molecule TAG-1 by anti-GD3 ganglioside monoclonal antibody.J Neurochem. 2001 Sep;78(5):1162-7. doi: 10.1046/j.1471-4159.2001.00515.x. J Neurochem. 2001. PMID: 11553690
-
A novel sphingomyelin/cholesterol domain-specific probe reveals the dynamics of the membrane domains during virus release and in Niemann-Pick type C.FASEB J. 2017 Apr;31(4):1301-1322. doi: 10.1096/fj.201500075R. Epub 2016 Aug 4. FASEB J. 2017. PMID: 27492925
-
Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts.J Cell Biol. 1990 Aug;111(2):429-42. doi: 10.1083/jcb.111.2.429. J Cell Biol. 1990. PMID: 2380243 Free PMC article.
-
Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy.Biochim Biophys Acta. 2014 May;1841(5):720-6. doi: 10.1016/j.bbalip.2013.07.003. Epub 2013 Jul 13. Biochim Biophys Acta. 2014. PMID: 23860017 Review.
-
Cholesterol interactions with ceramide and sphingomyelin.Chem Phys Lipids. 2016 Sep;199:26-34. doi: 10.1016/j.chemphyslip.2016.04.002. Epub 2016 Apr 27. Chem Phys Lipids. 2016. PMID: 27132117 Review.
Cited by
-
Evidence for the Involvement of Lipid Rafts and Plasma Membrane Sphingolipid Hydrolases in Pseudomonas aeruginosa Infection of Cystic Fibrosis Bronchial Epithelial Cells.Mediators Inflamm. 2017;2017:1730245. doi: 10.1155/2017/1730245. Epub 2017 Dec 3. Mediators Inflamm. 2017. PMID: 29333001 Free PMC article.
-
Direct interaction, instrumental for signaling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells.J Lipid Res. 2015 Jan;56(1):129-41. doi: 10.1194/jlr.M055319. Epub 2014 Nov 23. J Lipid Res. 2015. PMID: 25418321 Free PMC article.
-
Sphingolipids and plasma membrane hydrolases in human primary bronchial cells during differentiation and their altered patterns in cystic fibrosis.Glycoconj J. 2020 Oct;37(5):623-633. doi: 10.1007/s10719-020-09935-x. Epub 2020 Jul 14. Glycoconj J. 2020. PMID: 32666337 Free PMC article.
-
Evidence for coordination of lysosomal (ASMase) and plasma membrane (NSMase2) forms of sphingomyelinase from mutant mice.FEBS Lett. 2012 Nov 16;586(22):4002-9. doi: 10.1016/j.febslet.2012.09.039. Epub 2012 Oct 6. FEBS Lett. 2012. PMID: 23046545 Free PMC article.
-
β-Glucocerebrosidase Deficiency Activates an Aberrant Lysosome-Plasma Membrane Axis Responsible for the Onset of Neurodegeneration.Cells. 2022 Jul 29;11(15):2343. doi: 10.3390/cells11152343. Cells. 2022. PMID: 35954187 Free PMC article.
References
-
- Prinetti A., Chigorno V., Prioni S., Loberto N., Marano N., Tettamanti G., Sonnino S. 2001. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J. Biol. Chem. 276: 21136–21145 - PubMed
-
- Prinetti A., Chigorno V., Tettamanti G., Sonnino S. 2000. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J. Biol. Chem. 275: 11658–11665 - PubMed
-
- Valsecchi M., Mauri L., Casellato R., Prioni S., Loberto N., Prinetti A., Chigorno V., Sonnino S. 2007. Ceramide and sphingomyelin species of fibroblasts and neurons in culture. J. Lipid Res. 48: 417–424 - PubMed
-
- Prinetti A., Loberto N., Chigorno V., Sonnino S. 2009. Glycosphingolipid behaviour in complex membranes. Biochim. Biophys. Acta. 1788: 184–193 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

