HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma
- PMID: 19820691
- PMCID: PMC3092591
- DOI: 10.1038/modpathol.2009.139
HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma
Abstract
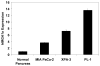
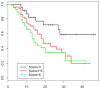
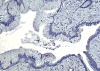
Although pancreatic ductal adenocarcinoma is a common and almost uniformly fatal cancer, little is known about the molecular events that lead to tumor progression. The high-mobility group A1 (HMGA1) protein is an architectural transcription factor that has been implicated in the pathogenesis and progression of diverse human cancers, including pancreatic ductal adenocarcinoma. In this study, we investigated HMGA1 expression in pancreatic ductal adenocarcinoma cell lines and surgically resected tumors to determine whether it could be a marker for more advanced disease. By real-time quantitative RT-PCR, we measured HMGA1a mRNA in cultured pancreatic ductal adenocarcinoma cell lines and found increased levels in all cancer cells compared with normal pancreatic tissue. To investigate HMGA1 in primary human tumors, we performed immunohistochemical analysis of 125 cases of pancreatic adenocarcinoma and 99 precursor lesions (PanIN 1-3). We found nuclear staining for HMGA1 in 98% of cases of pancreatic adenocarcinoma, but only 43% of cases of PanIN precursor lesions. Moreover, HMGA1 immunoreactivity correlates positively with decreased survival and advanced tumor and PanIN grade. These results suggest that HMGA1 promotes tumor progression in pancreatic ductal adenocarcinoma and could be a useful biomarker and rational therapeutic target in advanced disease.
Conflict of interest statement
No relevant financial or other disclosures or conflicts of interest.
Figures




References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. - PubMed
-
- Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690–2698. - PubMed
-
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

