Structural insights into RNA processing by the human RISC-loading complex
- PMID: 19820710
- PMCID: PMC2845538
- DOI: 10.1038/nsmb.1673
Structural insights into RNA processing by the human RISC-loading complex
Abstract
Targeted gene silencing by RNA interference (RNAi) requires loading of a short guide RNA (small interfering RNA (siRNA) or microRNA (miRNA)) onto an Argonaute protein to form the functional center of an RNA-induced silencing complex (RISC). In humans, Argonaute2 (AGO2) assembles with the guide RNA-generating enzyme Dicer and the RNA-binding protein TRBP to form a RISC-loading complex (RLC), which is necessary for efficient transfer of nascent siRNAs and miRNAs from Dicer to AGO2. Here, using single-particle EM analysis, we show that human Dicer has an L-shaped structure. The RLC Dicer's N-terminal DExH/D domain, located in a short 'base branch', interacts with TRBP, whereas its C-terminal catalytic domains in the main body are proximal to AGO2. A model generated by docking the available atomic structures of Dicer and Argonaute homologs into the RLC reconstruction suggests a mechanism for siRNA transfer from Dicer to AGO2.
Figures


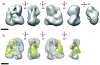
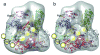
References
-
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. - PubMed
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
-
- Rand TA, Petersen S, Du FH, Wang XD. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. - PubMed
-
- Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

