Humans and evolutionary and ecological forces shaped the phylogeography of recently emerged diseases
- PMID: 19820723
- PMCID: PMC2794044
- DOI: 10.1038/nrmicro2219
Humans and evolutionary and ecological forces shaped the phylogeography of recently emerged diseases
Abstract
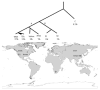
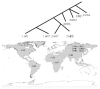
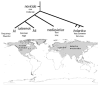
The development of human civilizations and global commerce has led to the emergence and worldwide circulation of many infectious diseases. Anthrax, plague and tularaemia are three zoonotic diseases that have been intensely studied through genome characterization of the causative species and phylogeographical analyses. A few highly fit genotypes in each species represent the causative agents for most of the observed disease cases. Together, mutational and selective forces create highly adapted pathogens, but this must be coupled with ecological opportunities for global expansion. This Review describes the distributions of the bacteria that cause anthrax, plague and tularaemia and investigates the forces that created clonal structures in these species.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

