Oral contraceptive pill for primary dysmenorrhoea
- PMID: 19821293
- PMCID: PMC7154221
- DOI: 10.1002/14651858.CD002120.pub3
Oral contraceptive pill for primary dysmenorrhoea
Update in
-
Combined oral contraceptive pill for primary dysmenorrhoea.Cochrane Database Syst Rev. 2023 Jul 31;7(7):CD002120. doi: 10.1002/14651858.CD002120.pub4. Cochrane Database Syst Rev. 2023. PMID: 37523477 Free PMC article.
Abstract
Background: Dysmenorrhoea (painful menstrual cramps) is common. Combined OCPs are recommended in the management of primary dysmenorrhoea.
Objectives: To determine the effectiveness and safety of combined oral contraceptive pills for the management of primary dysmenorrhoea.
Search strategy: We conducted electronic searches for randomised controlled trials (RCTs) in the Cochrane Menstrual Disorders and Subfertility Group Register of controlled trials CENTRAL, CCTR, MEDLINE, EMBASE, and CINAHL (first conducted in 2001, updated on 5 November 2008).
Selection criteria: RCTs comparing all combined OCPs with other combined OCPs, placebo, no management, or management with nonsteroidal anti-inflammatories (NSAIDs) were considered.
Data collection and analysis: Twenty three studies were identified and ten were included. Six compared the combined OCP with placebo and four compared different dosages of combined OCP.
Main results: One study of low dose oestrogen and four studies of medium dose oestrogen combined OCPs compared with placebo, for a combined total of 497 women, reported pain improvement. For the outcome of pain relief across the different OCPs the pooled OR suggested benefit with OCPs compared to placebo (7 RCTs: Peto OR 2.01 [95% CI 1.32, 3.08]).The Chi-squared test for heterogeneity showed there is significant heterogeneity with an I(2) statistic of 64% and a significant chi-square test (14.06, df=5, p=0.02). A sensitivity analysis removing the studies with inadequate allocation concealment suggested significant benefit of treatment with the pooled OR of 2.99 (95% CI 1.76, 5.07) and heterogeneity no longer statistically significant and I(2) statistic of 0%.Three studies reported adverse effects (Davis 2005; Hendrix 2002; GPRG 1968) The adverse effects were nausea, headaches and weight gain. Two studies reported if women experienced any side effect and no evidence of an effect was found (3 RCTs: OR = 1.45 (95% 0.71, 2.94). There was no evidence of statistical heterogeneity.There were no studies identified that compared combined OCP versus non steroidal anti-inflammatory drugsThere was no evidence of a difference for the pooled studies for 3rd generation pro gestagens (OR = 1.11 (95% CI 0.79 - 1.57)). For the 2nd generation versus 3rd generation the OR was 0.44 (95% CI 0.23-0.84) suggesting benefit of the 3rd generation OCP but this was for a single study (Winkler 2003).
Authors' conclusions: There is limited evidence for pain improvement with the use of the OCP (both low and medium dose oestrogen) in women with dysmenorrhoea. There is no evidence of a difference between different OCP preparations.
Conflict of interest statement
Helen Roberts has received funding from Schering, Wyeth, Pharmaco Organanon, and Pharmacia Upjohn in the past.
Figures

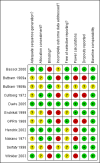
















Update of
-
Oral contraceptive pill as treatment for primary dysmenorrhoea.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD002120. doi: 10.1002/14651858.CD002120.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD002120. doi: 10.1002/14651858.CD002120.pub3. PMID: 19370576 Updated.
References
References to studies included in this review
Bassol 2000 {published data only}
-
- Bassol S, Alvarado A, Celis C, Cravioto MC, Peralta O, Montano R, et al. Latin American experience with two low‐dose oral contraceptives containing 30µg ethinylestradiol/75µg gestodene and 20µg ethinyl estradiol/150µg desogestrel. Contraception 2000;62(3):131‐5. - PubMed
Buttram 1969a {published data only}
-
- Buttram VC, Kaufman RH. Primary dysmenorrhoea: combination vs sequential therapy. Texas Medicine 1969;65(8):52‐5. - PubMed
Buttram 1969b {published data only}
-
- Buttram VC, Kaufman RH. Primary dysmenorrhoea: combination vs sequential therapy. Texas Medicine 1969;65(8):52‐5. - PubMed
Cullberg 1972 {published data only}
-
- Cullberg J. Mood changes and menstrual symptoms with different gestagen/estrogen combinations. Acta Psychiatrica Scandinavia Supplementum 1972;236:1‐86. - PubMed
Davis 2005 {published and unpublished data}
-
- Davis AR, Westhoff C, O'Connell K, Callagher N. Oral contraceptives for dysmenorrhea in adolescent girls: A randomized trial. Obstetrics & Gynecology 2005;106(1):97‐104. - PubMed
-
- O'Connell K, David AR, Kerns J. Oral contraceptives: side effects and depression in adolescent girls. Contraception 2007;75:299‐304. [10.1016/j.contraception.2006.09.008] - PubMed
Endrikat 1999 {published data only}
-
- Endrikat J, Dusterberg B, Ruebig A, Gerlinger C, Strowitzki T. Comparison of efficacy, cycle control, and tolerability of two low‐dose oral contraceptives in a multicenter clinical study. Contraception 1999;60(5):269‐74. - PubMed
GPRG 1968 {published data only}
-
- General Practitioner Research Group. Dysmenorrhoea unrelieved by an oral contraceptive. Practitioner 1968;200:856‐9. - PubMed
Hendrix 2002 {published data only}
-
- Hendrix SL, Alexander NJ. Primary dysmenorrhea treatment with a desogestrel‐containing low‐dose oral contraceptive. Contraception 2002;66(6):393‐9. - PubMed
Nakano 1971 {published data only}
-
- Nakano R, Takemura H. Treatment of function dysmenorrhoea: a double‐blind study. Acta Obstetrica et Gynaecologica Japonica 1971;18(1):41‐4. - PubMed
Serfaty 1998 {published data only}
-
- Serfaty D, Vree ML. A comparison of the cycle control and tolerability of two ultra low‐dose oral contraceptives containing 20µg ethinyl estradiol and either 150µg desogestrel and 75µg gestodene. The European Journal of Contraception and Reproductive Health Care 1998;3(4):179‐89. - PubMed
Winkler 2003 {published and unpublished data}
-
- Winkler UH, Ferguson H, Mulders JAPA. Cycle control, quality of life and acne with two low‐dose oral contraceptives containing 20µg ethinylestradiol. Contraception 2004;69(6):469‐76. - PubMed
References to studies excluded from this review
Creatsas 1998 {published data only}
-
- Creatsas G, Cardamakis E, Deligeoroglou E, Hassan E, Tzingounis V. Tenoxicam versus lynestrenol‐ethinyl estradiol treatment of dysfunctional uterine bleeding cases during adolescence. Journal of Paediatric & Adolescent Gynaecology 1998;11(4):177‐180. - PubMed
Foidart 2000 {published data only}
-
- Foidart JM, Wuttke W, Bouw GM, Gerlinger C, Heithecker R. A comparative investigation of contraceptive reliability, cycle control and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. The European Journal of Contraception and Reproductive Health Care 2000;5(2):124‐34. - PubMed
Iannotti 1991 {published data only}
-
- Iannotti G, DeFalco F, Paladini D, Ferraro F, Ragone R, Facchiano C, Ferrara A. Estrogen‐progestagen and prostaglandin inhibitors in the treatment of primary dysmenorrhoea [Estroprogestinici ed antiprostaglandinici nelle terapia della dismenorrea primaria]. Giornale Italiano di Ostetricia e Ginecologia 1991;13(2):87‐90.
Karasawa 1968 {published data only}
-
- Karasawa Y. Treatment of dysmenorrhoea with a mixed preparation of norethindrone and mestranol (S‐3800C). Sanfujinka No Jissai ‐ Practice of Gynecology & Obstetrics 1968;17(10):913‐8. - PubMed
Kaunitz 2000 {published data only}
-
- Kaunitz AM. Efficacy, cycle control, and safety of two triphasic oral contraceptives: Cyclessa (Desogestrel/Ethinyl Estradiol) and Ortho‐Novum 7/7/7(Norethindrone/Ethinyl Estradiol): A randomised clinical trial. Contraception 2000;61(5):295‐302. - PubMed
Kremser 1971 {published data only}
-
- Kremser E, Mitchell GM. Treatment of primary dysmenorrhoea with a combined type oral contraceptive ‐ a double blind study. Journal of the American College Health Association 1971;19(3):195‐6. - PubMed
Kristjansdottir 2000 {published data only}
-
- Kristjansdottir J, Johansson EDB, Ruusuvaara L. The cost of the menstrual cycle in young Swedish women. The European Journal of Contraception and Reproductive Health Care 2000;5(2):152‐6. - PubMed
Kwiecien 2003 {published data only}
-
- Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low‐dose oral contraceptive: a randomised trial. Contraception 2003;67(1):9‐13. - PubMed
La Guardia 2003 {published data only}
-
- LaGuardia KD, Shangold G, Fisher A, Friedman A, Kafrissen M, the Norgestimate Study Group. Efficacy, safety and cycle control of five oral contraceptive regimens containing norgestimate and ethinyl estradio. Contraception 2003;67(6):431‐437. 2003;67(6):431‐7. - PubMed
Matthews 1968 {published data only}
-
- Matthews AE, Clarke JE. Double‐blind trial of a sequential oral contraceptive (Sequens) in the treatment of dysmenorrhoea.. Journal of Obstetrics & Gynaecology of the British Commonwealth 1968;75(11):1117‐22. - PubMed
Moore 1999 {published data only}
-
- Moore C, Feichtinger W, Klinger G, Melliger U, Spona J, Walter F, Winkler UH, Zahradnik HP. Clinical findings with the dienogest‐containing oral contraceptive Valette(TM). Drugs of Today 1999;35(Suppl. C):53‐68.
Reisman 1999 {published data only}
-
- Reisman H, Deborah M, Gast MJ. A multicenter randomized comparison of cycle control and laboratory findings with oral contraceptive agents containing 100µg levonorgestrel with 20µg ethinyl estradiol or triphasic norethindrone with ethinyl estradiol. American Journal of Obstetrics & Gynecology 1999;181(5 part 2):S45‐S52. - PubMed
Tallian 1994 {published data only}
-
- Tallian F. Therapeutic possibilities using newer types of combined pills [Terapias lehetosegek ujabb tipusu kombinalt hormontablettakkal]. Magyar Noorvosok Lapja 1994;57:189‐92.
Additional references
Brill 1991
-
- Brill K, Norpoth T, Schnitker J, Albring M. Clinical experience with a modern low‐dose oral contraceptive in almost 100,000 users. Contraception 1991;43(2):101‐110. - PubMed
Chan 1981
-
- Chan WY, Dawood MY, Fuchs F. Prostaglandins in primary dysmenorrhea: Comparison of prophylactic and non prophylactic treatment with ibuprofen and use of oral contraceptives. American Journal of Medicine 1981;70:535‐41. - PubMed
Coco 1999
-
- Coco AS. Primary Dysmenorrhoea. American Family Physician 1999;60(2):489‐96. - PubMed
Dawood 1984
-
- Dawood MY. Ibuprofen and dysmenorrhea. American Journal of Medicine 1984;77(1A):87‐94. - PubMed
Dawood 1990
-
- Dawood MY. Dysmenorrhea. Clinical Obstetrics and Gynecology 1990;33(1):168‐78. - PubMed
Dawood 2006
-
- Dawood MY. Primary Dysmenorrhea ‐ Advances in pathogenesis and management. Obstetrics Gynaecology 2006;108:428‐41. - PubMed
Gauthier 1992
-
- Gauthier A, Upmalis D, Dain M. Clinical evaluation of a new triphasic oral contraceptive: norgestimate and ethinyl estradiol. Acta Obstetricia et Gynecologica Scandinavica Supplement 1992;71(Suppl 156):27‐32. - PubMed
Goldzieher 1971
-
- Goldzieher JW, Moses LE, Averkin E, Scheel C, Taber BZ. A placebo‐controlled double‐blind crossover investigation of the side effects attributed to oral contraceptives. Fertility and Sterility 1971;22(9):609‐23. - PubMed
Goldzieher 1995
-
- Goldzieher JW, Zamah NM. Oral contraceptive side effects: Where's the beef?. Contraception 1995;52:327‐35. - PubMed
Karnaky 1975
-
- Karnaky KJ. Development of the oral contraceptives. American Journal of Obstetrics and Gynecology 1975;123(7):771‐772. - PubMed
Melzack 1994
-
- Melzack R, Katz J. Pain measurement in persons in pain. In: PD Wall, R Melzack editor(s). Textbook of Pain. 3rd Edition. London: Churchill Livingstone, 1994:337‐351.
Milsom 1984
-
- Milsom I, Andersch B. Effect of various oral contraceptive combinations on dysmenorrhea. Gynaecologic and Obstetric Investigation 1984;17:284‐292. - PubMed
Milsom 1990
-
- Milsom I, Sundell G, Andersch B. The influence of different combined oral contraceptives on the prevalence and severity of dysmenorrhea. Contraception 1990;42(5):497‐506. - PubMed
Smith 1993
-
- Smith RP. Cyclic pain and dysmenorrhea. Obstetrics and Gynecology Clinics of North America 1993;20(4):752‐764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

