Homocysteine lowering interventions for preventing cardiovascular events
- PMID: 19821378
- PMCID: PMC4164174
- DOI: 10.1002/14651858.CD006612.pub2
Homocysteine lowering interventions for preventing cardiovascular events
Update in
-
Homocysteine-lowering interventions for preventing cardiovascular events.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD006612. doi: 10.1002/14651858.CD006612.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2015 Jan 15;1:CD006612. doi: 10.1002/14651858.CD006612.pub4. PMID: 23440809 Updated.
Abstract
Background: Cardiovascular disease such as coronary artery disease, stroke and congestive heart failure, is a leading cause of death worldwide. A postulated risk factor is elevated circulating total homocysteine (tHcy) levels which is influenced mainly by blood levels of cyanocobalamin (vitamin B12), folic acid (vitamin B9) and pyridoxine (vitamin B6). There is uncertainty regarding the strength of association between tHcy and the risk of cardiovascular disease.
Objectives: To assess the clinical effectiveness of homocysteine-lowering interventions (HLI) in people with or without pre-existing cardiovascular disease.
Search strategy: We searched The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (issue 3 2008), MEDLINE (1950 to August 2008), EMBASE (1988 to August 2008), and LILACS (1982 to September 2, 2008). We also searched in Allied and Complementary Medicine (AMED; 1985 to August 2008), ISI Web of Science (1993 to August 2008), and the Cochrane Stroke Group Specialised Register (April 2007). We hand searched pertinent journals and the reference lists of included papers. We also contacted researchers in the field. There was no language restriction in the search.
Selection criteria: We included randomised clinical trials (RCTs) assessing the effects of HLI for preventing cardiovascular events with a follow-up period of 1 year or longer. We considered myocardial infarction and stroke as the primary outcomes. We excluded studies in patients with end-stage renal disease.
Data collection and analysis: We independently performed study selection, risk of bias assessment and data extraction. We estimated relative risks (RR) for dichotomous outcomes. We measured statistical heterogeneity using I(2). We used a random-effects model to synthesise the findings.
Main results: We included eight RCTs involving 24,210 participants with a low risk of bias in general terms. HLI did not reduce the risk of non-fatal or fatal myocardial infarction, stroke, or death by any cause (pooled RR 1.03, 95% CI 0.94 to 1.13, I(2) = 0%; pooled RR 0.89, 95% CI 0.73 to 1.08, I(2) = 15%); and pooled RR 1.00 (95% CI 0.92 to 1.09, I(2): 0%), respectively.
Authors' conclusions: Results from available published trials suggest that there is no evidence to support the use of HLI to prevent cardiovascular events.
Figures


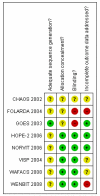




References
References to studies included in this review
-
- Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation. 2002;106(Suppl II):741.
-
- Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, Tijssen JG, et al. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. International Journal of Cardiology. 2004;93:175–9. [MEDLINE: 14975544] - PubMed
-
-
*
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. Journal of the American College of Cardiology. 2003;41:2105–13. [MEDLINE: 12821232] - PubMed
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91:1213–4. [MEDLINE: 16103563] - PMC - PubMed
-
-
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine. 2006;354:1567–77. [MEDLINE: 16531613] - PubMed
-
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. New England Journal of Medicine. 2006;354:1578–88. [MEDLINE: 16531614] - PubMed
References to studies excluded from this review
-
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–6. - PubMed
-
- Nevado J. [accessed Oct 12th 2007];Folate Intervention in Non-ST elevation myocardial infarction and unstable angina: a randomised placebo-controlled trial. 2006 http://controlled-trials.com/ISRCTN30249553/homocysteine.
-
- Lange H, Suryapranata H, De Luca G, Börner C, Dille J, Kallmayer K, et al. Folate therapy and in-stent restenosis after coronary stenting. New England Journal of Medicine. 2004;350:2673–81. [MEDLINE: 15215483] - PubMed
-
- Lonn E. Homocysteine in the prevention of ischemic heart disease, stroke and venous thromboembolism: therapeutic target or just another distraction? Current Opinion in Hematology. 2007;14:481–7. [MEDLINE: 17934354] - PubMed
-
- Neal B, MacMahon S, Ohkubo T, Tonkin A, Wilcken D, PACIFIC Study Group Dose-dependent effects of folic acid on plasma homocysteine in a randomized trial conducted among 723 individuals with coronary heart disease. European Heart Journal. 2002;23:1509–15. [MEDLINE: 12395803] - PubMed
References to ongoing studies
-
- MacMahon M, Kirkpatrick C, Cummings CE, Clayton A, Robinson PJ, Tomiak RH, et al. A pilot study with simvastatin and folic acid/vitamin B12 in preparation for the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Nutrition, Metabolism, and Cardiovascular Diseases. 2000;10:195–203. - PubMed
-
- Galan P, de Bree A, Mennen L, Potier de Courcy G, Preziozi P, Bertrais S, et al. Background and rationale of the SU.FOL.OM3 study: double-blind randomized placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. The Journal of Nutrition, Health & Aging. 2003;7:428–35. - PubMed
-
- The VITATOPS (Vitamins to Prevent Stroke) Trial The VITATOPS (Vitamins to Prevent Stroke) Trial: rationale and design of an international, large, simple, randomised trial of homocysteine-lowering multivitamin therapy in patients with recent transient ischaemic attack or stroke. Cerebrovascular Diseases. 2002;13:120–6. - PubMed
Additional references
-
- Abahji TN, Nill L, Ide N, Keller C, Hoffmann U, Weiss N. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Archives of Medical Research. 2007;38:411–6. - PubMed
-
- Anderson JL, Jensen KR, Carlquist JF, Bair TL, Horne BD, Muhlestein JB. Effect of folic acid fortification of food on homocysteine-related mortality. American Journal of Medicine. 2004;116:158–64. [MEDLINE: 14749159] - PubMed
-
- B-Vitamin Treatment Trialists’ Collaboration Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. American Heart Journal. 2006;151:282–7. - PubMed
-
- Beł towski J. Protein homocysteinylation: a new mechanism of atherogenesis? Postȩ py higieny i medycyny doś wiadczalnej. 2005;59:392–404. [MEDLINE: 16106241] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

