Abatacept for rheumatoid arthritis
- PMID: 19821401
- PMCID: PMC6464777
- DOI: 10.1002/14651858.CD007277.pub2
Abatacept for rheumatoid arthritis
Abstract
Background: Abatacept inhibits the co-stimulation of T cells and disrupts the inflammatory chain of events that leads to joint inflammation, pain, and damage in rheumatoid arthritis.
Objectives: To assess the efficacy and safety of abatacept in reducing disease activity, pain, and improving function in people with rheumatoid arthritis.
Search strategy: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 1), MEDLINE (from 1966), EMBASE (from 1980), ACP Journal Club (from 2000), and Biosis Previews (from 1990) in March 2007 and December 2008. We contacted authors of included studies and the abatacept manufacturer.
Selection criteria: Randomized controlled trials comparing abatacept alone, or in combination with disease-modifying anti-rheumatic drugs (DMARDs) or biologics, to placebo or other DMARDs or biologics in patients with moderate to severe rheumatoid arthritis.
Data collection and analysis: Two authors independently assessed search results and risk of bias, and extracted data. We obtained adverse event data from trials, long-term extension studies, and regulatory agencies.
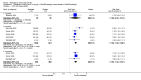
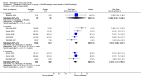
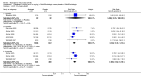
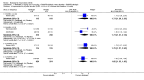
Main results: Seven trials with 2908 patients were included. Compared with placebo, patients in the abatacept group were 2.2 times more likely to achieve an ACR 50 response at one year (RR 2.21, 95% confidence interval (CI) 1.73 to 2.82) with a 21% (95% CI 16% to 27%) absolute risk difference between groups. The number needed to treat to achieve an ACR 50 response was 5 (95% CI 4 to 7). Significant improvements in physical function and a reduction in disease activity and pain were found in abatacept-treated patients compared to placebo. One RCT found abatacept significantly slowed the radiographic progression of joint damage at 12 months compared to placebo, although it is not clear what the clinical relevance of this difference may be. There may be a risk of attrition bias. Total adverse events were greater in the abatacept group (RR 1.05, 95% CI 1.01 to 1.08). Other harm outcomes were not significant with the exception of a greater number of serious infections at 12 months in the abatacept group (Peto odds ratio 1.91 (95% CI 1.07 to 3.42). Serious adverse events were increased when abatacept was given in combination with other biologics (RR 2.30, 95% CI 1.15 to 4.62).
Authors' conclusions: There is moderate-level evidence that abatacept is efficacious and safe in the treatment of rheumatoid arthritis. Abatacept should not be used in combination with other biologics to treat rheumatoid arthritis. The withdrawal and toxicity profile appears acceptable at the present time but further long-term studies and post-marketing surveillance are required to assess harms and sustained efficacy.
Conflict of interest statement
None known. This systematic review did not receive specific funding.
Figures
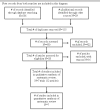
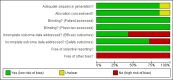
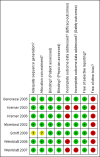
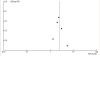





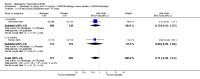


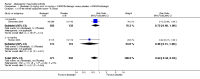




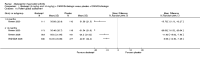








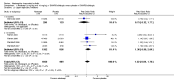


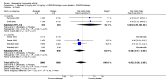

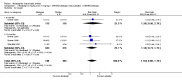

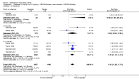

























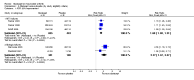
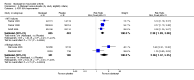
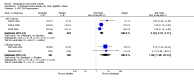
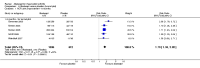


Update of
References
References to studies included in this review
Genovese 2005 {published data only}
-
- Cole J, Li T, Lin P, MacLean R, Wallenstein GV. Treatment impact on estimated medical expenditure and job loss likelihood in rheumatoid arthritis: re‐examining quality of life outcomes from a randomized placebo‐controlled clinical trial with abatacept. Rheumatology 2008;47(7):1044‐50. - PubMed
-
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005;353(11):1114‐23 [erratum appears in New England Journal of Medicine 2005;353(21):2311]. - PubMed
-
- Westhovens R, Cole JC, Li T, Martin M, Maclean R, Lin P, et al. Improved health‐related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti‐TNF therapy in a double‐blind, placebo‐controlled, multicentre randomized clinical trial. Rheumatology 2006;45(10):1238‐46. - PubMed
Kremer 2003 {published and unpublished data}
-
- Emery P, Kosinski M, Li T, Martin M, Williams GR, Becker JC, et al. Treatment of rheumatoid arthritis patients with abatacept and methotrexate significantly improved health‐related quality of life. Journal of Rheumatology 2006;33(4):681‐9. - PubMed
-
- Kremer JM, Dougados M, Emery P, Durez P, Sibilia J, Shergy W, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve‐month results of a phase IIb, double‐blind, randomized, placebo‐controlled trial. Arthritis & Rheumatism 2005;52(8):2263‐71 [erratum appears in Arthritis & Rheumatism 2005;52(10):3321]. - PubMed
-
- Kremer JM, Westhovens R, Leon M, Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. New England Journal of Medicine 2003;349(20):1907‐15. - PubMed
Kremer 2006 {published and unpublished data}
-
- Kremer J, Genant H, Moreland L, Russel A, Emery P, Abud‐Mendoza C, et al. Effects of abatacept in patients with methotrexate‐resistant active rheumatoid arthritis. Annals of Internal Medicine 2006;144:865‐76. - PubMed
Moreland 2002 {published and unpublished data}
-
- Moreland LW, Alten R, BF, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose‐finding, double‐blind, placebo‐controlled clinical trial evaluating CTLA‐4Ig and LEA29Y eighty‐five days after the first infusion. Arthritis & Rheumatism 2002;46(6):1470‐9. - PubMed
Schiff 2008 {published and unpublished data}
-
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, Saldate C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Annals of the Rheumatic Diseases 2008;67(8):1096‐103. - PMC - PubMed
Weinblatt 2006 {published and unpublished data}
-
- Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease‐modifying antirheumatic drugs: a one‐year randomized, placebo‐controlled study. Arthritis & Rheumatism 2006;54(9):2807‐16. - PubMed
Weinblatt 2007 {published and unpublished data}
References to studies excluded from this review
Alten 2006 {published data only}
-
- Alten R. Long‐term therapy with the selective costimulation modulator CTLA4lg in rheumatoid arthritis [German]. Zeitschrift fur Rheumatologie 2006;65(3):252‐3. - PubMed
FDA 2005 {published data only}
-
- FDA Briefing document: ORENCIA (abatacept) BLA 125118/0. FDA website (http://www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005‐4170B1_02_01‐FDA‐Ab....) (accessed 22 February 2007).
Genant 2008 {published data only}
Genovese 2004 {published data only}
-
- Genovese M, Luggen M, et al. Efficacy and safety of abatacept (CTLA41g), a selective co‐stimulation modulator, in rheumatoid arthritis patients not responding adequately to anti‐TNF‐alpha therapy: results of the phase III ATTAIN (Abatacept trial in treatment of Anti‐TNF INadequate responders). Arthritis & Rheumatism 2004;50(12):4103.
Genovese 2005a {published data only}
-
- Genovese M, Luggen M, et al. Sustained improvements through 18 months with abatacept in rheumatoid arthritis patients with an inadequate response to anti‐TNF therapy. Arthritis & Rheumatism 2005;52(12):4064.
Genovese 2008b {published data only}
-
- Genovese M, Schiff M, et al. Efficacy and safety of the selective co‐stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti‐tumour necrosis factor therapy. Annals of the Rheumatic Diseases 2008;67(4):547‐54. - PubMed
Haggerty 2007 {published data only}
-
- Haggerty HG, Abbott MA, et al. Evaluation of immunogenicity of the T cell costimulation modulator abatacept in patients treated for rheumatoid arthritis. Journal of Rheumatology 2007;34(12):2365‐73. - PubMed
Hassett 2007 {published data only}
-
- Hassett A, Maclean J, et al. An observational evaluation of psychological well‐being in rheumatoid arthritis in the attain trial for ABATACEPT. Annals of the Rheumatic Diseases 2007;66 (Suppl.2):278.
Kremer 2008 {published data only}
-
- Kremer JM, Genant HK, et al. Results of a two‐year follow up study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis & Rheumatism 2008;58(4):953‐63. - PubMed
Kruger 2005 {published data only}
-
- Kruger K. Selective inhibition of T‐cell activation with fusion protein CTLA4lg as a new treatment option for rheumatoid arthritis [German]. Zeitschrift fur Rheumatologie 2005;64(1):40‐1. - PubMed
Li 2008 {published data only}
-
- Li T, Gignac M. Decreased external home help use with improved clinical status in rheumatoid arthritis: an exploratory analysis of the abatacept in inadequate responders to methotrexate (AIM) trial. Clinical Therapeutics 2008;30(4):734‐48. - PubMed
NHS 2004 {published data only}
-
- Abatacept (CTLA4Ig) for rheumatoid arthritis unresponsive to current therapies: new and emerging technology briefing. National Horizon Scanning Centre, University of Birmingham (http://www.pcpoh.bham.ac.uk/publichealth/horizon/PDF_files/2004reports/A...) (accessed 2 March 2007) 2004.
Taylor 2006 {published data only}
-
- Taylor PC. Is abatacept an effective treatment for patients with RA who do not respond to other anti‐TNF treatments?. Nature Clinical Practice Rheumatology 2006;2(3):128‐9. - PubMed
Teng 2005 {published data only}
-
- Teng GG, Turkiewicz AM, Moreland LW. Abatacept: a costimulatory inhibitor for treatment of rheumatoid arthritis. Expert Opinion on Biological Therapy 2005;5(9):1245‐54. - PubMed
Weisman 2006 {published data only}
-
- Weisman MH, Durez P, Hallegua D, Aranda R, Becker JC, Nuamah I, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. Journal of Rheumatology 2006;33(11):2162‐6. - PubMed
Wells 2008 {published data only}
-
- Wells G, Li T, et al. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Annals of the Rheumatic Diseases 2008;67(2):260‐5. - PubMed
Additional references
ACR 2007
-
- ACR Hotline. http://www.rheumatology.org/publications/hotline/0506newdrugs.asp (accessed 3 February 2007).
ARC 2005
-
- ARC Epidemiology Unit. http://www.arc.org.uk/about_arth/bigpic.htm#3 ‐ARC Epidemiology Unit (accessed 3 February 2007).
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism 1988;31:315‐24. - PubMed
Badley 2003
-
- Badley E, DesMeules M, eds. Arthritis in Canada. An ongoing challenge. Ottawa: Health Canada, 2003.
Bhandari 2004
Blumenauer 2002
Blumenauer 2003
-
- Blumenauer B, Judd M, Cranney A, Burls A, Coyle D, Hochberg M, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004525.] - PubMed
Boers 2006
-
- Boers M. Abatacept in rheumatoid arthritis: a new branch on the “biologics” tree (editorial). Annals of the Rheumatic Diseases 2006;144:933‐5. - PubMed
Buch 2008
Cates 2004 [Computer program]
-
- Cates C. Visual Rx 2.0 NNT Calculator. Dr Chris Cates EBM Website www.nntonline.net, 2004.
Consort 2001
-
- Moher D, Schulz K, Altman DG, the CONSORT Group. Revised recommendations for improving the quality of reports of parallel group randomized trials. Lancet 2001;357:1191‐4. - PubMed
DAS 2009
-
- DAS‐Score.nl. Home of the DAS. Disease Activity Score in rheumatoid arthritis. http://www.das‐score.nl/www.das‐score.nl/ (accessed 24 July 2009).
EMEA 2007
-
- Orencia Scientific Discussion document ‐ European Medicines Agency, European Public Assessment Report. http://www.emea.europa.eu/humandocs/PDFs/EPAR/orencia/H‐701‐en6.pdf 2007.
Farrar 2001
-
- Farrar J, Young Jr. JP, LaMoreauxb L, Werthb JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001;94:149‐58. - PubMed
FDA 2007
-
- FDA Orencia Label. FDA Center for Drug Evaluation and Research http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... 13 March 2007 (accessed 1 April 2009).
Felson 1995
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis & Rheumatism 1995;38:727‐35. - PubMed
Helmick 2008
-
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh Kent C, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis and Rheumatism 2008;58(1):15‐25. - PubMed
Higgins 2003
Massarotti 2008
-
- Massarotti E. Clinical and patient‐reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clinical Therapeutics 2008;30(3):429‐42. - PubMed
Navarro‐Sarabia 2005
Orencia 2007
-
- Orencia website. http://www.orencia.com/orencia/channels/content.jsp?BV_UseBVCookie=Yes&a... (accessed 1 February 2007).
Osiri 2002
PMPRB 2004
-
- Report on New Patented Drugs ‐ Humira. Patented Medicines Prices Review Board (http://www.pmprb‐cepmb.gc.ca/english/view.asp?x=478&pf=1) (accessed 24 July 2009) 2004.
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration.. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration., 2008.
Ruiz 2009
Salliot 2009
Schultz 1995
-
- Schultz K, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schumacher 1993
-
- Schumacher HR Jr, Klippel JH, Koopman WJ. Chapter 10. Rheumatoid Arthritis. Primer on the rheumatic diseases. 10th Edition. Arthritis Foundation, 1993.
Sibilia 2007
-
- Sibilia J, Westhovens R. Safety of T‐cell co‐stimulation modulation with abatacept in patients with rheumatoid arthritis. Clinical Experimental Rheumatology 2007;25 (Suppl. 46):S46‐S56. - PubMed
Suarez‐Almazor 1998
-
- Suarez‐Almazor ME, Belseck E, Shea BJ, Tugwell P, Wells G. Methotrexate for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000957.]
Suarez‐Almazor 1998b
-
- Suarez‐Almazor ME, Belseck E, Shea BJ, Tugwell P, Wells G. Sulfasalazine for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000958]
Suarez‐Almazor 2000
-
- Suarez‐Almazor ME, Belseck E, Shea BJ, Homik JJEH, Wells G, Tugwell P. Antimalarials for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000959] - PubMed
Tubach 2007
Van der Heijde 1993
-
- Heijde DM, Hof M, Riel PL, Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. Journal of Rheumatology 1993;20:579‐81. - PubMed
Van Gestel 1996
-
- Gestel AM, Prevoo ML, Hof MA, Rijswijk MH, Putte LB, Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organisation/International League Against Rheumatism criteria. Arthritis & Rheumatism 1996;39:34‐40. - PubMed
Woolf 2004
-
- Woolf A, Åkesson K, Compston J, et al. European action towards better musculoskeletal health ‐ a public health strategy to reduce the burden of musculoskeletal conditions; the Bone and Joint Decade, 2004. The Bone & Joint Decade, Department of Orthopedics, University Hospital, SE‐221 85 LUND, Sweden, 2004.
Yazici 2008
-
- Yazici Y, Adler NM, Yazici H. Most tumour necrosis factor inhibitor trials in rheumatology are undeservedly called 'efficacy and safety' trials: a survey of power considerations. Rheumatology 2008;47(7):1054‐7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

