Risperidone dose for schizophrenia
- PMID: 19821422
- PMCID: PMC11972846
- DOI: 10.1002/14651858.CD007474.pub2
Risperidone dose for schizophrenia
Abstract
Background: Risperidone is a widely used antipsychotic drug for people with schizophrenia. It is important to get a balance between gaining the most positive effects for the least negative outcomes. The optimal dose of risperidone is the focus of this review.
Objectives: To determine risperidone dose response relationships for schizophrenia and schizophrenia-like psychoses.
Search strategy: We searched the Cochrane Schizophrenia Groups Trials Register (July 2008) for all relevant references.
Selection criteria: All relevant randomised controlled clinical trials (RCTs).
Data collection and analysis: Two review authors independently extracted data and resolved disagreement by discussion with a third member of the team. When insufficient data were provided, we contacted the study authors. For homogenous dichotomous data we calculated fixed-effect relative risk (RR) and 95% confidence intervals (CI) on an intention-to-treat basis. For continuous data, we calculated weighted mean differences (MD).
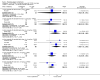
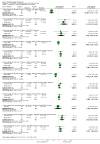
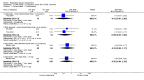
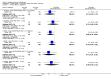
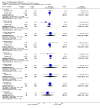
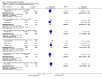
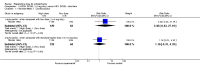
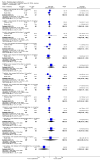
Main results: A consistent finding when risperidone ultra low doses (<2 mg/day) were compared with other doses (short-term data) was that more people left early because of insufficient response (n=456, 1 RCT, RR when compared with standard-low (>==4-<6 mg/day) 12.48 CI 1.43 to 4.30). The insufficient response for this low dose is reflected in measures of mental state. When low doses (>==2-<4 mg/day) are used and compared with standard-higher doses (>==6-<10 mg/day) and the high dose range (>==10 mg/day), more people left early because of insufficient response (>==4-<6 mg/day: n=173, 2 RCTs, RR 4.05 CI 1.09 to 15.07; >==10 mg/day: n=173, 2 RCTs, RR 1.92 CI 1.36 to 2.70). For the outcome of 'no clinically important improvement' results favour standard-higher doses (n=272, 2 RCTs, RR 2.26 CI 0.81 to 6.34). When low doses are compared with other higher doses, we found no differences in terms of cardiovascular, CNS, endocrine or gastrointestinal adverse effects. Unspecified EPS were more frequent with the higher doses (>==10 mg: n=262, 2 RCTs, RR 0.45 CI 0.24 to 0.84). One trial did find that endpoint scores on PANSS significantly favoured a low dose when compared with >==4-6 mg/day (n=124, 1 RCT, MD -12.40 CI -17.01 to -7.79). When >==4-<6 mg/day is compared with high doses, less people left early (n=677, 1 RCT, RR leaving any reason 0.74 CI 0.54 to 1.00; n=677, 1 RCT, RR due to adverse effects 0.56 CI 0.32 to 0.97). >==4-<6 mg/day was no worse than >==6-<10 mg/day for 'no clinically important improvement' (n=39, 1 RCT, RR on CGI-I 0.79 CI 0.29 to 2.17). People allocated >==4-<6 mg/day had more movement disorders than those on a low dose (n=124 1 RCT, RR 2.28 CI 1.67 to 3.11). When >==6-<10 mg/day is compared with standard-lower doses and a high dose range, there is no significant difference in terms of proportions leaving early. >==6-<10 mg/day is better than a low dose for 'no clinical important improvement' (n=172, 2 RCTs, RR 0.76 CI 0.61 to 0.94). Overall >==6-<10 mg/day caused less problems especially in EPS when compared with >==10mg/day (n=261, 2 RCTs, RR unspecified EPS 0.56 CI 0.31 to 0.99). When a high dose was compared with a low dose less people left early (n=70, 1 RCT, RR 0.43 CI 0.26 to 0.71) but not when compared with a standard-lower dose (n=677, 1 RCT, RR leaving due to adverse event 1.78 CI 1.03 to 3.09). >==10 mg/day was better than a low dose in terms of 'no clinical important improvement' (n=257, 2 RCTs, RR 0.64 CI 0.50 to 0.82), but worse than a standard-higher dose (>==6-<10 mg/day: n=255, 2 RCTs, RR 1.22 CI 1.00 to 1.51). >==10 mg/day caused more unspecified EPS adverse effects and any drug for adverse events when compared with a standard-higher dose and with a low dose.
Authors' conclusions: There is still lack of strong evidence for an optimal dose for clinical practice. The quality of trials suggests that an over estimate of effect is likely and we think this is most probably for the mid-range doses. One such dose (standard-lower dose range, 4-<6 mg/day) does seem optimal for clinical response and adverse effects. Weak evidence suggests that low doses (>==2-<4 mg/day) may be of value for people in their first episode of illness. High doses (>==10 mg/day) did not confer any advantage over any other dose ranges and caused more adverse effects, especially for movement disorders. Ultra low dose (<2 mg/day) seemed useless. We advise the use of dosages from low dose to standard-lower dose for different kinds of individual patients. Future trials should focus on specific populations, e.g. those in their first episode, with acute exacerbation, in relapse or refractory to treatment, and should also test the optimal dose of risperidone over a longer period of time and in the community.
Conflict of interest statement
None known.
Figures
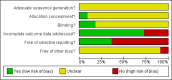





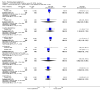
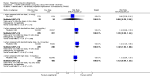
















































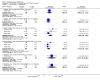


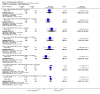
Update of
References
References to studies included in this review
Agid 2007 {published data only}
-
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, Kapur S. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response‐‐a double‐blind PET study in schizophrenia. Neuropsychopharmacology 2007;32(6):1209‐15. [MEDLINE: ] - PubMed
-
- Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. American Journal of Psychiatry 2007;164(4):630‐7. [CINAHL: 2009568249; EMBASE: 2007204841; MEDLINE: ] - PubMed
Chouinard 1993 {published data only}
-
- Anderson C, Clark WR, True J, Ereshefsky L. Risperidone, a novel antipsychotic and weight change. Pharmacotherapy 1993;13:292.
-
- Anderson C, True J, Ereshefsky L, Miller A. Risperidone clinical efficacy: role of the metabolite 9‐hydroxy‐risperidone. Psychopharmacology Bulletin 1994;30(4):88.
-
- Anderson CB, True JE, Ereshefsky L, Miller AL, Peters BL, Velligan DI. Risperidone dose, plasma levels and response. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; 1993 May 22‐27; San Francisco, California, USA. 1993.
-
- Chouinard G. Effects of risperidone in tardive dyskinesia: an analysis of the Canadian multicenter risperidone study. Journal of Clinical Psychopharmacology 1995; Vol. 15, issue Suppl 1:S36‐44. [MEDLINE: ] - PubMed
-
- Chouinard G, Albright PS. Economic and health state utility determinations for schizophrenic patients treated with risperidone or haloperidol. Journal of Clinical Psychopharmacology 1997;17(4):298‐307. [MEDLINE: ] - PubMed
Klieser 1995 {published data only}
-
- Heinrich K. Risperidone versus clozapine in acute schizophrenia. Proceedings of the 1st International Risperidone Investigators' Meeting; 1992 Mar 9‐10; Paris, France. 1992:27‐8.
-
- Heinrich K, Klieser E, Lehmann E, Kinzler E. Experimental comparison of the efficacy and compatibility of clozapine and risperidone in acute schizophrenia. Clinical Neuropharmacology 1992;15(Suppl 1 Pt B):375B.
-
- Heinrich K, Klieser E, Lehmann E, Kinzler E. Experimental comparison of the efficacy and compatibility of risperidone and clozapine in acute schizophrenia. Proceedings of the 17th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1990 Sep 10‐14; Kyoto, Japan. 1991.
-
- Heinrich K, Klieser E, Lehmann E, Kinzler E, Hruschka H. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double blind, randomized trial. Progress in Neuropsychopharmacology and Biological Psychiatry 1994;18(1):129‐37. [MEDLINE: ] - PubMed
-
- Klieser E, Kinzler E. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double‐blind, randomised trial. Clinical report 1991. - PubMed
Klieser 1996 {published data only}
-
- Klieser E, Lehmann E, Heinrich K. Risperidone in comparison with various treatments of schizophrenia. In: Kane JM editor(s). Serotonin in Antipsychotic Treatment. Mechanisms and Clinical Practice. New York, USA: Marcel Dekker Inc., 1996:331‐43.
Lane 2001 {published data only}
-
- Lane HY, Chang WH, Chiu CC, Huang MC, Lee SH, Chen JY. A pilot double‐blind, dose‐comparison study of risperidone in drug‐naive, first‐episode schizophrenia. Journal of Clinical Psychiatry 2001;62(12):994‐5. [MEDLINE: ] - PubMed
Marder 1994 {published data only}
-
- Anderson C, Clark WR, True J, Ereshefsky L. Risperidone, a novel antipsychotic and weight change. Pharmacotherapy 1993;13:292.
-
- Anderson C, True J, Ereshefsky L, Miller A. Risperidone clinical efficacy: role of the metabolite 9‐hydroxy‐risperidone. Psychopharmacology Bulletin 1994;30(4):88.
-
- Anderson CB, True JE, Ereshefsky L, Miller AL, Peters BL, Velligan DI. Risperidone dose, plasma levels and response. Proceedings of the 146th Annual Meeting of the American Psychiatric Association; 1993 May 22‐27; San Francisco, California, USA. 1993.
-
- Blaeser‐Kiel G. Successful therapy with risperidone in schizophrenic negative syndrome [Schizophrenes Negativsyndrom. Risperidon Erfolgreich]. TW Neurologie Psychiatrie 1994;8(11):614‐5. [EMBASE: 1994342404]
-
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. Journal of Clinical Psychopharmacology 1995;15(4):243‐9. [MEDLINE: ] - PubMed
Peuskens 1992 {published data only}
-
- Bechelli LP, Moreno R, Versiani M, Caetano D, Mari J, AcioliA. Risperidone x haloperidol: brazilian results of an international trial. Proceedings of the 9th World Congress of Psychiatry; 1993 Jun 6‐12; Rio de Janeiro, Brazil. 1993.
-
- Crocket G, T, Mortimer AM, Livingston MG, Cookson JC, Jauhar P, Luthra JS, Batchelor DH, Hammond GL. Risperidone versus haloperidol in the treatment of chronic schizophrenic patients: UK centres in an international dose finding study. Proceedings of the 18th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1992 Jun 28 ‐ Jul 2; Nice, France 1992.
-
- Boer JA, Westenberg HGM, Jansen AAI. Risperidone in the treatment of chronic schizophrenia: a double blind comparative study with haloperidol. Proceedings of the 18th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1992 Jun 28 ‐ Jul 2; Nice, France. 1992.
-
- Duarte A, Borda JT. Risperidone vs haloperidol in schizophrenic patients. Argentine results. Proceedings of the 9th World Congress of Psychiatry; 1993 Jun 6‐12; Rio de Janeiro, Brazil. 1993:335.
-
- Heylen SL, Gelders YG. Risperidone, a new antipsychotic with serotonin 5‐HT2 and dopamine D2 antagonistic properties. Clinical Neuropharmacology 1992;15(Suppl 1):180A‐1A. - PubMed
Wei 2006 {published data only}
-
- Wei C, Liu H, Guo R, Fu C. Relationship of plasma risperidone concentration and the efficacy in treatment of first‐episode schizophrenia after a middle or low dosage risperidone administration. Chin J Clin Pharmacol Ther 2006;11(11):1313‐16.
Wu 2001 {published data only}
-
- Wu T, Li Y, Li M, Efficacy of very low dosage of risperidone in the treatment of first‐episode schizophrenia. Journal of Clinical Psychological Medicine 2001;11(3):152‐4. [MEDI0109]
Ying 2004 {published data only}
-
- Ying L, Li Z, Wu Y. Comparative studies on different dosage risperidone in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases 2004;10(3):158‐160, 171.
Zhang 2004 {published data only}
-
- Zhang H, Liu ZC, Wang GH. Efficacy and safety of different fixed dose of risperidone in treatment of first‐episode schizophrenia. Chinese Journal of Psychiatry 2004;37(1):26‐9. [MEDI0404]
References to studies excluded from this review
Alvarez 2006 {published data only}
-
- Alvarez E, Ciudad A, Olivares JM, Bousono M, Gomez JC. A randomized, 1‐year follow‐up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. Journal of Clinical Psychopharmacology 2006;26(3):238‐49. - PubMed
-
- Gurpegui M, Alvarez E, Bousono M, Ciudad A, Gomez JC, Olivares JM. Effect of olanzapine or risperidone treatment on some cognitive functions in a one‐year follow‐up of schizophrenia outpatients with prominent negative symptoms. European Neuropsychopharmacology 2007;17(11):725‐34. - PubMed
Borison 1992a {published data only}
-
- Borison R. Risperidone versus haloperidol in acute exacerbations of chronic schizophrenia. Proceedings of the 1st International Risperidone Investigators' Meeting; 1992 Mar 9‐10; Paris, France. USA, 1992. [LMD87729]
Bouchard 1998 {published data only}
-
- Bouchard RH. A comparative longitudinal study of risperidone versus classic neuroleptic drugs in the treatment of schizophrenia: 24 Months observation [Etude comparative longitudinale de la risperidone versus les neuroleptiques classiques dans le traitemante de la schizophrenie: 24 mois d'observation]. Encephale 2002;28(5 II):31‐2. - PubMed
-
- Bouchard RH, Demers MF, Merette C, Pourcher E. Classical neuroleptics (CNLP) vs risperidone (RIS) interim 12 months efficacy analysis of a long‐ term, naturalistic study in schizophrenics. Proceedings of the 21st Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1998 Jul 12‐16; Glasgow, UK. 1998.
-
- Bouchard RH, Merette C, Demers MFAU, Roy‐Gagnon MHAU, =Quebec Study Group. Risperidone versus classical neuroleptics ‐ MSc, preliminary results of a prospective naturalistic one‐year study. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30 ‐ Jun 4; Toronto, Ontario, Canada. 1998.
-
- Bouchard RH, Merette C, Pourcher E, Demers MF, Villeneuve J, Roy Gagnon MH, Gauthier Y, Cliche D, Labelle A, Filteau MJ, Roy MA, Maziade M, =Quebec Schizophrenia Study Group. Longitudinal comparative study of risperidone and conventional neuroleptics for treating patients with schizophrenia. Journal of Clinical Psychopharmacology 2000;20(3):295‐304. - PubMed
-
- Bouchard RH, Pourcher E, Merette C, Demers MF, Villeneuve J, Roy MA, Gauthier Y, Labelle A, Cliche D, Maziade M, =Quebec Schizophrenia Study Group. Risperidone advantages in chronic schizophrenic patients: a 1‐year effectiveness study conference abstract. Schizophrenia Research 1999;36(1‐3):271.
Brecher 1998 {published data only}
-
- Berry S, Martinez R, Myers J E, Mahmoud R. Serum prolactin in schizophrenia. Proceedings of the 7th World Congress of Biological Psychiatry; 2001 Jul 1‐6; Berlin, Germany. 2001.
-
- Berry S, Martinez RA, Myers JE, Mahmoud R. Serum prolactin in schizophrenia. European Neuropsychopharmacology 2001;11(3):257.
-
- Berry SA, Gudelsky GA, Mahmoud RA. Serum prolactin levels in schizophrenia. Biological Psychiatry 2001;49(8):22S.
-
- Berry SA, Martinez RA, Gudelsky GA, Mahmoud R, Myers J. Serum prolactin levels in schizophrenia. Schizophrenia Research 2001;49(1,2):280‐1.
-
- Berry SA, Martinez RA, Gudelsky GA, Myers JE, Mahmoud RA. Serum prolactin in schizophrenia. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10‐14; San Juan, Puerto Rico. 2000.
Eerdekens 2004 {published data only}
-
- Eerdekens M, Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long‐acting risperidone in schizophrenia. Schizophrenia Research 2004;70(1):91‐100. - PubMed
Emsley 1999 {published data only}
-
- Emsley RA, =Risperidone Working Group. Risperidone in the treatment of first episode psychotic patients: a double blind multicenter study. Schizophrenia Bulletin 1999;25(4):721‐9. - PubMed
Harrigan 2004 {published data only}
-
- Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTC, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology 2004;24(1):62‐9. - PubMed
Kopala 2003 {published data only}
-
- Kopala L, Rabinowitz J, Davidson M. Extra‐pyramidal signs and symptoms (eps) in recent onset schizophrenia: a comparison of risperidone and haloperidol. European Neuropsychopharmacology 2003;13(4):S338.
-
- Rabinowitz J, Davidson M, Kopala L. A method for examining efficacy by dosage in flexible dose clinical trials. Schizophrenia Research 2004;67(1):150.
Lacro 2001 {published data only}
-
- Lacro J. Antipsychotic treatment in late life schizophrenia. CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html) (accessed 19th February 2001). [CRISP: Grant Number ‐ 5R29MH56938‐04]
Meltzer 2008 {published data only}
-
- Meltzer H, Peters P, Elkis H, Ruschel S, Rosenthal M, Mills R. Co‐therapy with pimavanserin and risperidone 2 mg provides an improved clinical profile. Schizophrenia Research 2008;98:16.
Potkin 2006 {published data only}
-
- Potkin SG, Gharabawi GM, Greenspan AJ, Mahmoud R, Kosik‐Gonzalez C, Rupnow MFT, Bossie CA, Davidson M, Burtea V, Zhu Y, Trivedi JK. A double‐blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencing an acute exacerbation requiring hospitalization. Schizophrenia Research 2006;85(1‐3):254‐65. - PubMed
Wang 2002 {published data only}
-
- Wang Y, Zuo J, Xun Z. A clinical study on the effects of risperidone in treating senile patients with schizophrenia. Tianjin Pharmacy 2002;6(3):56‐7.
Xin 2001 {published data only}
-
- Xin X‐F, Du B‐C, Zeng Z‐X. A controlled clinical study of risperidone and low dose of clozapine in treating schizophrenia. Herald of Medicine 2001;20(8):501‐2.
Zhong 2005 {published data only}
-
- Zhong K, Harvey P, Brecher M, Sweitzer D. A randomized double ‐ blind study of quetiapine and risperidone in the treatment of schizophrenia. Schizophrenia Bulletin 2005;31:508.
References to studies awaiting assessment
Chen 2001e {published data only}
-
- Chen FB, Yu HY, Liu GY, Zhang SY, Gao ZQ. Plasma drug level of risperidone and its clinical significance. Chinese New Drugs Journal 2001;10(6):450‐3.
Merlo 2002 {published data only}
-
- Merlo MCG. Hofer H, Fabry MG, Marder SR. Improvement of cognitive functions in acute first‐episode psychosis treated with risperidone. Schizophrenia Research 2002;53(3 Suppl 1):27.
References to ongoing studies
Lieberman 2001a {published data only}
-
- Lieberman JA. Risperidone and clozapine in chronic schizophrenia. CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html) (accessed 19th February 2001). [CRISP: Grant Number ‐ 5R10MH53551‐05]
Additional references
Altman 1996
Andreasen 1983
-
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry 1983;39:784‐8. - PubMed
Bland 1997
BNF 2008
-
- British National Formulary (BNF). Risperidone. http://www.bnf.org/bnf/bnf/55/3248.htm (accessed 25 July 2008).
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Buckley 2008
-
- Buckley PF, Correll CU. Strategies for dosing and switching antipsychotics for optimal clinical management. Journal of Clinical Psychiatry 2008;69(Suppl 1):4‐17. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330(10):681‐90. - PubMed
Chan 2005
Chouinard 1980
-
- Chouinard G, Ross‐Chouinard A, Annable L, Jones BD. Extrapyramidal symptom rating scale. Canadian Journal of Neurological Science 1980;7:233.
Chouinard 1993 b
-
- Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Arnott W. A Canadian multicenter placebo‐controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. Journal of Clinical Psychopharmacology 1993;13(1):25‐40. - PubMed
Conley 2003
-
- Conley RR, Kelly DL. Pharmacologic treatment of schizophrenia. 2nd Edition. Caddo,OK: Professional communications,Inc., 2003.
Davis 2004
-
- Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. Journal of clinical psychopharmacology 2004;24(2):192‐208. [PUBMED: 15206667] - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiol 2002;31(1):140‐9. - PubMed
Ezewuzie 2006
-
- Ezewuzie N, Taylor D. Establishing a dose‐response relationship for oral risperidone in relapsed schizophrenia. Journal of psychopharmacology 2006;20(1):86‐90. [PUBMED: 16174679] - PubMed
FDA 2005
-
- U.S. Food, Drug Administration (FDA). Janssen pharmaceutica products, l.p. risperdal (risperidone) tablets/oral solution, risperdal m‐tab (risperidone) orally disintegrating tablets. http://www.fda.gov/cder/foi/label/2005/020272s042,020588s030,021444s016,... (accessed 25 July 2005).
FDA 2008
-
- U.S. Food, Drug Administration. FDA news (June 30, 2008): fda approves first generic risperidone to treat psychiatric conditions. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01855.html (accessed date 5 August 2008).
Green 2000
-
- Green B. Focus on Risperidone. Current Medical Research and Opinion 2000;16(2):57‐65. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1970
-
- Guy W, Bonato RR, eds. Clinical Global Impressions. Manual for the ECDEU Assessment Battery 2. Revised. National Institute of Mental Health, 1970.
Higgins 2003
Higgins 2005
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration. Chichester: John Wiley & Sons, Ltd, 2008.
Juni 2001
Kane 1988
-
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Archives of general psychiatry 1988;45(9):789‐96. [PUBMED: 3046553] - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Lehfeld 1997
-
- Lehfeld H, Erzigkeit H. The SKT‐‐a short cognitive performance test for assessing deficits of memory and attention. International Psychogeriatrics 1997;9:115‐21. - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Lingjerde 1987
-
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatrica Scandinavica 1987;334(Suppl):1‐100. - PubMed
Marder 1994 b
-
- Marder SR. Risperidone: efficacy. Journal of Clinical Psychiatry 1994;12:49‐52.
Marder 1997
-
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. Journal of Clinical Psychiatry 1997;58(12):538‐46. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Megens 1994
-
- Megens AA, Awouters FH, Schotte A, Meert TF, Dugovic C, Niemegeers CJ, Leysen JE. Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology 1994;114(1):9‐23. - PubMed
Melander 2003
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PUBMED: 11323066] - PubMed
Montgomery 2004
-
- Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, Holland R. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled clinical trials 2004;25(6):598‐612. [PUBMED: 15588746] - PubMed
Muller 1998
-
- Muller MJ, Wetzel H, Benkert O. Differential treatment effects of amisulpride versus flupentixol on latent dimensions of depressive and negative symptoms in acute schizophrenia. Schizophrenia Research 1998;29(1,2):157. - PubMed
Möller 2005
-
- Möller HJ. Risperidone: a review. Expert Opinion on Pharmacotherapy 2005;6(5):803‐18. - PubMed
NIMH 1985
-
- National Institute of Mental Health. Treatment Emergent Symptoms Scale ‐‐ write‐in (TESS). Psychopharmacology Bulletin 1985;21:1069‐73.
Nyberg 1993
-
- Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson. 5‐HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology 1993;110(3):265‐72. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Peuskens 1995
-
- Peuskens J, =Risperidone Study Group. Risperidone in the treatment of patients with chronic schizophrenia: a multi‐national, multi‐centre, double‐blind, parallel‐group study versus haloperidol. British Journal of Psychiatry 1995;166:712‐26. - PubMed
Rust 1989
-
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989.
Simpson 1970
-
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;212(Suppl):11‐9. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Waraich 2002
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. 2007.
Zhang 2004 a
-
- Zhang H, Liu ZC, Wang GH. Efficacy and safety of different fixed dose of risperidone in treatment of first‐episode schizophrenia. Chinese Journal of Psychiatry 2004;37(1):26‐9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

