Medical treatments for idiopathic thrombocytopenic purpura during pregnancy
- PMID: 19821437
- PMCID: PMC6718204
- DOI: 10.1002/14651858.CD007722.pub2
Medical treatments for idiopathic thrombocytopenic purpura during pregnancy
Abstract
Background: Idiopathic thrombocytopenic purpura (ITP) is a common hematologic disorder caused by immune-mediated thrombocytopenia. The magnitude of the maternal-fetal risk of ITP during pregnancy is controversial. Labour management of pregnant women with ITP remains controversial. Management of ITP during pregnancy is complex because of the disparity between maternal and fetal platelet counts.
Objectives: To assess the effectiveness and safety of corticosteroids, intravenous immunoglobulin, vinca alkaloids, danazol, dapsone, and any other types of pharmacological treatments for the treatment of idiopathic thrombocytopenic purpura during pregnancy.
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (February 2009), LILACS (1982 to 8 February 2009), ClinicalTrials.gov (8 February 2009), Current Controlled Trials (16 February 2009), Google Scholar (16 February 2009) and ongoing and unpublished trials cited in the reference lists of relevant articles.
Selection criteria: Randomised controlled trials (RCTs) on any medical treatments for idiopathic thrombocytopenia purpura during pregnancy.
Data collection and analysis: Two review authors independently evaluated methodological quality and extracted trial data. Any disagreement was resolved by discussion or by consulting a third review author.
Main results: This review included one RCT in which 38 women (41 pregnancies) were randomised, with only 26 women (28 pregnancies) being analysed.This RCT comparing the effect of betamethasone (1.5 mg/day) with no medication found no statistically significant difference in neonatal thrombocytopenia (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.62 to 2.05) and neonatal bleeding (RR 1.00, 95% CI 0.24 to 4.13). Review authors conducted an intention-to-treat analysis which showed similar findings: RR 1.18, 95% CI 0.57 to 2.45 and RR 1.05, 95% CI 0.24 to 4.61, respectively. Maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage were not studied by this RCT.
Authors' conclusions: Current evidence indicates that compared to no medication, betamethasone did not reduce the risk of neonatal thrombocytopenia and neonatal bleeding in ITP during pregnancy. There is insufficient evidence to support the use of betamethasone for treating ITP. This Cohrane review does not provide evidence about other medical treatments for ITP during pregnancy. This systematic review also identifies the need for well-designed, adequately powered randomised clinical trials for this medical condition during pregnancy. Unless randomised clinical trials provide evidence of a treatment effect and the trade off between potential benefits and harms are established, policy-makers, clinicians, and academics should not use betamethasone for ITP in pregnant women. Any future trials on medical treatments for treating ITP during pregnancy should test a variety of important maternal, neonatal or both outcome measures, including maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage.
Conflict of interest statement
In 2004 Arturo Martí‐Carvajal was employed by Eli Lilly to run a four hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007 Arturo Martí‐Carvajal was employed by Merck to run a four hour workshop 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Guiomar Peña‐Martí and Gabriella Comunián‐Carrasco: None known.
Figures
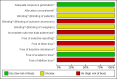
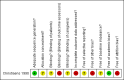








Update of
References
References to studies included in this review
Christiaens 1990 {published data only}
-
- Christiaens GCML, Nieuwenhuis HK, Dem Borne AEGK, Ouwehand WH, Helmerhorst FM, Dalen CM, et al. Idiopathic thrombocytopenic purpura in pregnancy: a randomized trial on the effect of antenatal low dose corticosteroids on neonatal platelet count. British Journal of Obstetrics and Gynaecology 1990; Vol. 97:893‐8. [MEDLINE: ] - PubMed
References to studies excluded from this review
Gill 2002 {published data only}
-
- Gill KK, Kelton JG. Management of idiopathic thrombocytopenic purpura in pregnancy. Seminars in Hematology 2000;37:275‐89. [MEDLINE: ] - PubMed
Lee 2002 {published data only}
-
- Lee LH. Idiopathic thrombocytopenia in pregnancy. Annals of the Academy of Medicine, Singapore 2002;31:335‐9. [MEDLINE: ] - PubMed
Additional references
Ali 2003
-
- Ali R, Ozkalemkas F, Ozçelik T, Ozkocaman V, Ozan U, Kimya Y, et al. Idiopathic thrombocytopenic purpura in pregnancy: a single institutional experience with maternal and neonatal outcomes. Annals of Hematology 2003;82(6):348‐52. [MEDLINE: ] - PubMed
Appel 2006
-
- Appel LJ. A primer on the design, conduct, and interpretation of clinical trials. Clinical Journal of the American Society of Nephrology 2006;1(6):1360‐7. [MEDLINE: ] - PubMed
Avilés‐Miranda 1983
-
- Avilés‐Miranda A, Ambríz Fernández R, Niz‐Ramos J, García‐Alonso A, Luna‐Olsen E, Sinco‐Angeles A, et al. Idiopathic thrombocytopenic purpura and pregnancy. Use of corticosteroids for the prevention of neonatal purpura. Revista de Investigación Clínica 1983;35:111‐4. [MEDLINE: ] - PubMed
BCSHGH 2003
-
- British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. British Journal of Haematology 2003;120:574‐96. [MEDLINE: ] - PubMed
Bhatla 1994
-
- Bhatla N, Buckshee K, Bhargava VL, Takkar D, Malhotra OP. Pregnancy with idiopathic thrombocytopenic purpura. Journal of the Association of Physicians of India 1994;42:105‐6. [MEDLINE: ] - PubMed
Borna 2002
-
- Borna S, Borna H, Khazardoost S. Maternal and neonatal outcomes in pregnant women with immune thrombocytopenic purpura. Archives of Iranian Medicine 2006;9:115‐8. [MEDLINE: ] - PubMed
Burrows 1990
-
- Burrows RF, Kelton JG. Thrombocytopenia at delivery: a prospective survey of 6715 deliveries. American Journal of Obstetrics and Gynecology 1990;162:731‐4. [MEDLINE: ] - PubMed
Bussel 2007
-
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine 2007;357:2237‐47. [MEDLINE: ] - PubMed
Byrne 1997
-
- Byrne JD, Incerpi MH, Goodwin TM. Idiopathic thrombocytopenic purpura in pregnancy treated with pulsed high‐dose oral dexamethasone. American Journal of Obstetrics and Gynecology 1997;177:468‐9. [MEDLINE: ] - PubMed
Capogna 2007
-
- Capogna G. Maternal and perinatal outcome in idiopathic thrombocytopenic purpura (ITP) with pregnancy. Obstetric Anesthesia Digest 2007;27:158‐9. [1536‐5395 ]
Chedraui 2003
-
- Chedraui PA, Hidalgo LA, San Miguel G. Fatal intracranial hemorrhage in a pregnant patient with autoimmune thrombocytopenic purpura. Journal of Perinatal Medicine 2003;31:526‐9. [MEDLINE: ] - PubMed
Cines 1982
-
- Cines DB, Dusak B, Tomaski A, Mennuti M, Schreiber AD. Immune thrombocytopenic purpura and pregnancy. New England Journal of Medicine 1982;306:826‐31. [MEDLINE: ] - PubMed
Cines 2005
-
- Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annual Review of Medicine 2005;56:425‐42. [MEDLINE: ] - PubMed
Cook 1991
-
- Cook RL, Miller RC, Katz VL, Cefalo RC. Immune thrombocytopenic purpura in pregnancy: a reappraisal of management. Obstetrics & Gynecology 1991;78:578‐83. [MEDLINE: ] - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001.
Devendra 2002
-
- Devendra K, Koh LP. Pregnancy in women with idiopathic thrombocytopaenic purpura. Annals of the Academy of Medicine, Singapore 2002;31:276‐80. [MEDLINE: ] - PubMed
Dufault 1957
-
- Dufault FX Jr, Kaye BM. Idiopathic thrombocytopenic purpura and pregnancy: report of a case treated with cortisone. Obstetrics & Gynecology 1957;9:228‐30. [MEDLINE: ] - PubMed
el Hajoui 2003
-
- Hajoui S, Nabil S, Khachani M, Kaddioui S, Alami MH, Bezad R, et al. Idiopathic thrombopenic purpura and pregnancy. La Tunisie Mèdicale 2003;81:213‐6. [MEDLINE: ] - PubMed
George 1996
-
- George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996;88:3‐40. [MEDLINE: ] - PubMed
George 1998
-
- George JN, Raskob GE. Idiopathic thrombocytopenic purpura: a concise summary of the pathophysiology and diagnosis in children and adults. Seminars in Hematology 1998;35(1 Suppl 1):5‐8. [MEDLINE: ] - PubMed
Gernsheimer 2007
-
- Gernsheimer T, McCraeb KR. Immune thrombocytopenic purpura in pregnancy. Current Opinion in Hematology 2007;14:574‐80. [MEDLINE: ] - PubMed
Gibson 1989
-
- Gibson J, Laird PP, Joshua DE, Child A, Harris J, Kronenberg H. Very high dose intravenous gammaglobulin in thrombocytopenia of pregnancy. Australian and New Zealand Journal of Medicine 1989;19:151‐3. [MEDLINE: ] - PubMed
Gross 1995
-
- Gross Z, Rodriguez JJ, Stalnaker BL. Vincristine for refractory autoimmune thrombocytopenic purpura in pregnancy. A case report. Journal of Reproductive Medicine 1995;40(10):739‐42. [MEDLINE: ] - PubMed
Harrington 1951
-
- Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. Journal of Laboratory and Clinical Medicine 1951;38:1‐10. [MEDLINE: ] - PubMed
Harrington 1953
-
- Harrington WJ, Sprague CC, Minnich V, Moore CV, Aulvin RC, Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Annals of Internal Medicine 1953;38:433‐69. [MEDLINE: ] - PubMed
Heys 1966
-
- Heys RF. Steroid therapy for idiopathic thrombocytopenic purpura during pregnancy. Obstetrics & Gynecology 1966;28:532‐42. [MEDLINE: ] - PubMed
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kalish 2001
-
- Kalish, RB, Christiaens GCML, Skupski DW, Bussel JB. Patterns of progression of immune thrombocytopenia purpura during pregnancy. American Journal of Obstetrics and Gynecology 2001;185(Suppl 6):S181.
Kimura 2001
-
- Kimura S, Kuroda J, Akaogi T, Hayashi H, Ogino Y, Kobayashi Y, et al. Treatment of steroid‐resistant idiopathic thrombocytopenic purpura in pregnancy with repeated high‐dose intravenous immunoglobulin. Haematologia 2001;31:263‐5. [MEDLINE: ] - PubMed
Kryc 1983
-
- Kryc JJ, Corrigan JJ Jr. Idiopathic thrombocytopenic purpura during pregnancy. A pediatric viewpoint. American Journal of Pediatric Hematology/Oncology 1983;5(1):21‐5. [MEDLINE: ] - PubMed
Kutter 2008
-
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet 2008;371:395‐403. [MEDLINE: ] - PubMed
Lush 2000
-
- Lush R, Iland H, Peat B, Young G. Successful use of dapsone in refractory pregnancy‐associated idiopathic thrombocytopenic purpura. Australian and New Zealand Journal of Medicine 2000;30:105‐7. [MEDLINE: ] - PubMed
Martí‐Carvajal 2003
-
- Martí‐Carvajal A. El Paciente con...Semiología y Clínica Hematológica. Valencia, Venezuela: Universidad de Carabobo, 2003.
McGee 2002
-
- McGee DC. Steroid use during pregnancy. Journal of Perinatal and Neonatal Nursing 2002;16:26‐39. [MEDLINE: ] - PubMed
Nisaratanaporn 2006
-
- Nisaratanaporn S, Sukcharoen N. Outcome of idiopathic thrombocytopenic purpura in pregnancy in King Chulalongkorn Memorial Hospital. Journal of the Medical Association of Thailand 2006;89(Suppl 4):S70‐S75. [MEDLINE: ] - PubMed
Perruca 2003
-
- Perruca PE, Munoz MP, Liendo PF, Ricci AP, Pérez CC, Domínguez CC, et al. Experience and management of idiopathic thrombocytopenic purpura during pregnancy [Experiencia y manejo del purpura trombocitopenico idiopatico durante el embarazo]. Revista Chilena de Obstetricia y Ginecología 2003;68(4):293‐9. [ISSN 0717‐7526]
Porta 2008
-
- Porta M, editor. A dictionary of epidemiology. New York: Oxford University Press, 2008. [ISBN 978‐0‐19‐531449‐6]
RevMan 2008 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Stasi 2008
-
- Stasi R, Evangelista ML, Amadori S. Novel thrombopoietic agents: a review of their use in idiopathic thrombocytopenic purpura. Drugs 2008;68:901‐12. [MEDLINE: ] - PubMed
Sukenik‐Halevy 2008
-
- Sukenik‐Halevy R, Ellis MH, Fejgin MD. Management of immune thrombocytopenic purpura in pregnancy. Obstetrical and Gynecological Survey 2008;63:182‐8. [MEDLINE: ] - PubMed
Suri 2006
-
- Suri V, Aggarwal N, Saxena S, Malhotra P, Varma S. Maternal and perinatal outcome in idiopathic thrombocytopenic purpura (ITP) with pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:1430‐5. [MEDLINE: ] - PubMed
Tampakoudis 1995
-
- Tampakoudis P, Billi H, Tantanassis T, Kalachanis I, Garipidou B, Sinakos Z, et al. Pregnancy and labor in idiopathic thrombocytopenic purpura. Geburtshilfe und Frauenheilkunde 1995;55:583‐6. [MEDLINE: ] - PubMed
Wang 2004
-
- Wang Q, Nie LL. Clinical analysis of 92 cases of pregnancy with idiopathic thrombocytopenic purpura. Zhonghua Fu Chan Ke Za Zhi. 2004;39:729‐32. [MEDLINE: ] - PubMed
Webert 2003
-
- Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11‐year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003;102:4306‐11. [MEDLINE: ] - PubMed
Widmer 1999
-
- Widmer A, Brühwiler H, Krause M, Lüscher KP. Severe autoimmune thrombocytopenia in pregnancy. Zeitschrift für Geburtshilfe und Neonatologie 1999;203:258‐60. [MEDLINE: ] - PubMed
Won 2005
Woods 1984a
-
- Woods VL Jr, Kurata Y, Montgomery RR, Tani P, Mason D, Oh EH, et al. Autoantibodies against platelet glycoprotein Ib in patients with chronic immune thrombocytopenic purpura. Blood 1984;164:156‐60. [MEDLINE: ] - PubMed
Woods 1984b
-
- Woods VL Jr, Oh EH, Mason D, McMillan R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood 1984;63:368‐75. [MEDLINE: ] - PubMed
Yamada 1999
-
- Yamada H, Kato EH, Kobashi G, Kishida T, Ebina Y, Kaneuchi M, et al. Passive immune thrombocytopenia in neonates of mothers with idiopathic thrombocytopenic purpura: incidence and risk factors. Seminars in Thrombosis and Hemostasis 1999;25:491‐6. [MEDLINE: ] - PubMed
Yildirim 2006
-
- Yildirim Y, Tinar S, Oner RS, Kaya B, Toz E. Gestational diabetes mellitus in patients receiving long‐term corticosteroid therapy during pregnancy. Journal of Perinatal Medicine 2006;34:280‐4. [MEDLINE: ] - PubMed
Zavala 2006
-
- Zavala D, Martí‐Carvajal A, Peña‐Martí G, Comunián G. Data extraction sheet to help manage the characteristics of included studies in Cochraen reviews. (www.cochrane.fcs.edu.ve/hrs). Valencia‐Venezuela: Universidad de Carabobo, 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

