Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection
- PMID: 19821442
- PMCID: PMC11651644
- DOI: 10.1002/14651858.CD008085
Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection
Abstract
Background: Blood loss during liver resection is one of the most important factors affecting the peri-operative outcomes of patients undergoing liver resection.
Objectives: To determine the benefits and harms of pharmacological interventions to decrease blood loss and to decrease allogeneic blood transfusion requirements in patients undergoing liver resections.
Search strategy: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until November 2008 for identifying the randomised trials.
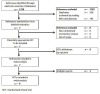
Selection criteria: We included all randomised clinical trials comparing various pharmacological interventions aimed at decreasing blood loss and allogeneic blood transfusion requirements in liver resection. Trials were included irrespective of whether they included major or minor liver resections, normal or cirrhotic livers, vascular occlusion was used or not, and irrespective of the reason for liver resection.
Data collection and analysis: Two authors independently identified trials for inclusion and independently extracted data. We analysed the data with both the fixed-effect and the random-effects models using RevMan Analysis. For each outcome we calculated the risk ratio (RR), mean difference (MD), or standardised mean difference with 95% confidence intervals (CI) based on intention-to-treat analysis or available case-analysis. For dichotomous outcomes with only one trial included under the outcome, we performed the Fisher's exact test.
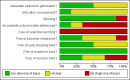
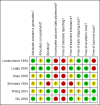
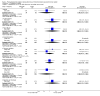
Main results: Six trials involving 849 patients satisfied the inclusion criteria. Pharmacological interventions included aprotinin, desmopressin, recombinant factor VIIa, antithrombin III, and tranexamic acid. One or two trials could be included under most comparisons. All trials had a high risk of bias. There was no significant difference in the peri-operative mortality, survival at maximal follow-up, liver failure, or other peri-operative morbidity. The risk ratio of requiring allogeneic blood transfusion was significantly lower in the aprotinin and tranexamic acid groups than the respective control groups. Other interventions did not show significant decreases of allogeneic transfusion requirements.
Authors' conclusions: None of the interventions seem to decrease peri-operative morbidity or offer any long-term survival benefit. Aprotinin and tranexamic acid show promise in the reduction of blood transfusion requirements in liver resection surgery. However, there is a high risk of type I (erroneously concluding that an intervention is beneficial when it is actually not beneficial) and type II errors (erroneously concluding that an intervention is not beneficial when it is actually beneficial) because of the few trials included, the small sample size in each trial, and the high risk of bias. Further randomised clinical trials with low risk of bias and random errors assessing clinically important outcomes such as peri-operative mortality are necessary to assess any pharmacological interventions aimed at decreasing blood loss and blood transfusion requirements in liver resections. Trials need to be designed to assess the effect of a combination of different interventions in liver resections.
Conflict of interest statement
None known.
Figures









References
References to studies included in this review
Lentschener 1999 {published data only}
-
- Lentschener C, Benhamou D, Mercier FJ, Boyer‐Neumann C, Naveau S, Smadja C, et al. Aprotinin reduces blood loss in patients undergoing elective liver resection. Anesthesia and Analgesia 1997;84(4):875‐81. - PubMed
-
- Lentschener C, Li H, Franco D, Mercier FJ, Lu H, Soria J, et al. Intraoperatively‐administered aprotinin and survival after elective liver resection for colorectal cancer metastasis: A preliminary study. Fibrinolysis & Proteolysis 1999;13(1):39‐45.
Lodge 2005 {published data only}
-
- Lodge JP, Jonas S, Oussoultzoglou E, Malago M, Jayr C, Cherqui D, et al. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo‐controlled, double‐blind clinical trial. Anesthesiology 2005;102(2):269‐75. - PubMed
Shao 2006 {published data only}
-
- Shao YF, Yang JM, Chau GY, Sirivatanauksorn Y, Zhong SX, Erhardtsen E, et al. Safety and hemostatic effect of recombinant activated factor VII in cirrhotic patients undergoing partial hepatectomy: a multicenter, randomized, double‐blind, placebo‐controlled trial. American Journal of Surgery 2006;191(2):245‐9. - PubMed
Shimada 1994 {published data only}
-
- Shimada M, Matsumata T, Kamakura T, Hayashi H, Urata K, Sugimachi K. Modulation of coagulation and fibrinolysis in hepatic resection: a randomized prospective control study using antithrombin III concentrates. Thrombosis Research 1994;74(2):105‐14. - PubMed
Wong 2003 {published data only}
-
- Wong AY, Irwin MG, Hui TW, Fung SK, Fan ST, Ma ES. Desmopressin does not decrease blood loss and transfusion requirements in patients undergoing hepatectomy. Canadian Journal of Anaesthesia 2003;50(1):14‐20. - PubMed
Additional references
Belghiti 1993
Boutron 2008
-
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148(4):W60‐W66. [PUBMED: 18283201] - PubMed
Chouker 2004
-
- Chouker A, Schachtner T, Schauer R, Dugas M, Lohe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. British Journal of Anaesthesia 2004;93(2):204‐11. - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Frilling 2005
-
- Frilling A, Stavrou GA, Mischinger HJ, Hemptinne B, Rokkjaer M, Klempnauer J, et al. Effectiveness of a new carrier‐bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial. Langenbecks Archives of Surgery 2005;390(2):114‐20. - PubMed
Gluud 2009
-
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2009, Issue 3. Art. No.: LIVER.
Gomez 2008
-
- Gomez D, Morris‐Stiff G, Wyatt J, Toogood GJ, Lodge JP, Prasad KR. Surgical technique and systemic inflammation influences long‐term disease‐free survival following hepatic resection for colorectal metastasis. Journal of Surgical Oncology 2008;98(5):371‐6. [PUBMED: 18646038] - PubMed
Gurusamy 2008
-
- Gurusamy KS, Osmani B, Sharma D, Davidson BR. Non‐surgical interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007338] - DOI
Gurusamy 2009a
Gurusamy 2009b
-
- Gurusamy KS, Pamecha V, Davidson BR. Topical haemostatic agents in liver resection. Cochrane Database of Systematic Reviews Issue under preparation.
Gurusamy 2009c
HES 2005
-
- Hospital Episode Statistics. Main operations. 3 character: 2004‐05. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&c... (accessed 19 March 2009).
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Ibrahim 2006
-
- Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplantation 2006;12(6):950‐7. - PubMed
Kitagawa 2001
-
- Kitagawa K, Taniguchi H, Mugitani T, Koh T, Obayashi T, Kunishima S, et al. Safety and advantage of perioperative autologous blood transfusion in hepatic resection for hepatocellular carcinoma. Anticancer Research 2001;21(5):3663‐7. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PUBMED: 11323066] - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
Poon 2001
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Shimada 1998
-
- Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. The British Journal of Surgery 1998;85(2):195‐8. - PubMed
Shinozuka 2000
-
- Shinozuka N, Koyama I, Arai T, Numajiri Y, Watanabe T, Nagashima N, et al. Autologous blood transfusion in patients with hepatocellular carcinoma undergoing hepatectomy. American Journal of Surgery 2000;179(1):42‐5. - PubMed
StatsDirect 2.7 [Computer program]
-
- StatsDirect Ltd. StatsDirect Statistical software Version 2.7.2. StatsDirect Ltd, 2008.
Strasberg 2000
-
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB Surgery 2000;2(3):333‐9.
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. - PubMed
Wang 2006
Wood 2008
Yoshimura 2004
-
- Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World Journal of Surgery 2004;28(10):982‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

