An adaptor domain-mediated autocatalytic interfacial kinase reaction
- PMID: 19821459
- PMCID: PMC2856317
- DOI: 10.1002/chem.200901345
An adaptor domain-mediated autocatalytic interfacial kinase reaction
Abstract
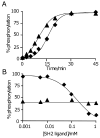
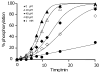
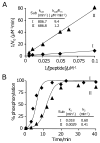
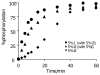
This paper describes a model system for studying the autocatalytic phosphorylation of an immobilized substrate by a kinase enzyme. This work uses self-assembled monolayers (SAMs) of alkanethiolates on gold to present the peptide substrate on a planar surface. Treatment of the monolayer with Abl kinase results in phosphorylation of the substrate. The phosphorylated peptide then serves as a ligand for the SH2 adaptor domain of the kinase and thereby directs the kinase activity to nearby peptide substrates. This directed reaction is intramolecular and proceeds with a faster rate than does the initial, intermolecular reaction, making this an autocatalytic process. The kinetic non-linearity gives rise to properties that have no counterpart in the corresponding homogeneous phase reaction: in one example, the rate for phosphorylation of a mixture of two peptides is faster than the sum of the rates for phosphorylation of each peptide when presented alone. This work highlights the use of an adaptor domain in modulating the activity of a kinase enzyme for an immobilized substrate and offers a new approach for studying biochemical reactions in spatially inhomogeneous settings.
Figures


References
-
- Shen-Orr SS, Milo R, Mangan S, Alon U. Nat Genet. 2002;31:64–68. - PubMed
-
- Mayer BJ, Hirai H, Sakai R. Curr Biol. 1995;5:296–305. - PubMed
- Pellicena P, Stowell KR, Miller TW. J Biol Chem. 1998;273:15325–15328. - PubMed
- Lewis LA, Chung CD, Chen Jy, Parnes JR, Moran M, Patel VP, Miceli MC. J Immunol. 1997;159:2292–2300. - PubMed
- Duyster J, Baskaran R, Wang JYJ. Proc Natl Acad Sci U S A. 1995;92:1555–1559. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

