Structural examination of the transient 3-aminotyrosyl radical on the PCET pathway of E. coli ribonucleotide reductase by multifrequency EPR spectroscopy
- PMID: 19821570
- PMCID: PMC4703294
- DOI: 10.1021/ja903879w
Structural examination of the transient 3-aminotyrosyl radical on the PCET pathway of E. coli ribonucleotide reductase by multifrequency EPR spectroscopy
Abstract
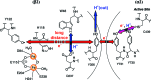
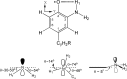
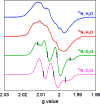
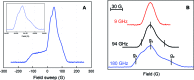
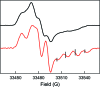
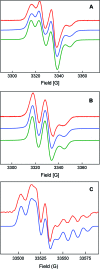
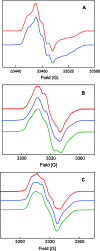
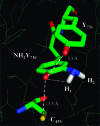
E. coli ribonucleotide reductase (RNR) catalyzes the conversion of nucleotides to deoxynucleotides, a process that requires long-range radical transfer over 35 A from a tyrosyl radical (Y(122)*) within the beta2 subunit to a cysteine residue (C(439)) within the alpha2 subunit. The radical transfer step is proposed to occur by proton-coupled electron transfer via a specific pathway consisting of Y(122) --> W(48) --> Y(356) in beta2, across the subunit interface to Y(731) --> Y(730) --> C(439) in alpha2. Using the suppressor tRNA/aminoacyl-tRNA synthetase (RS) methodology, 3-aminotyrosine has been incorporated into position 730 in alpha2. Incubation of this mutant with beta2, substrate, and allosteric effector resulted in loss of the Y(122)* and formation of a new radical, previously proposed to be a 3-aminotyrosyl radical (NH(2)Y*). In the current study [(15)N]- and [(14)N]-NH(2)Y(730)* have been generated in H(2)O and D(2)O and characterized by continuous wave 9 GHz EPR and pulsed EPR spectroscopies at 9, 94, and 180 GHz. The data give insight into the electronic and molecular structure of NH(2)Y(730)*. The g tensor (g(x) = 2.0052, g(y) = 2.0042, g(z) = 2.0022), the orientation of the beta-protons, the hybridization of the amine nitrogen, and the orientation of the amino protons relative to the plane of the aromatic ring were determined. The hyperfine coupling constants and geometry of the NH(2) moiety are consistent with an intramolecular hydrogen bond within NH(2)Y(730)*. This analysis is an essential first step in using the detailed structure of NH(2)Y(730)* to formulate a model for a PCET mechanism within alpha2 and for use of NH(2)Y in other systems where transient Y*s participate in catalysis.
Figures
References
-
- Stubbe J.; van der Donk W. A. Chem. Rev. 1998, 98, 705. - PubMed
-
- Jordan A.; Reichard P. Annu. Rev. Biochem. 1998, 67, 71. - PubMed
-
- Brown N. C.; Canellakis Z. N.; Lundin B.; Reichard P.; Thelander L. Eur. J. Biochem. 1969, 9, 561. - PubMed
-
- Thelander L. J. Biol. Chem. 1973, 248, 4591. - PubMed
-
- Stubbe J. J. Biol. Chem. 1990, 265, 5329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

