An NSAID-like compound, FT-9, preferentially inhibits gamma-secretase cleavage of the amyloid precursor protein compared to its effect on amyloid precursor-like protein 1
- PMID: 19821615
- PMCID: PMC4489158
- DOI: 10.1021/bi901237k
An NSAID-like compound, FT-9, preferentially inhibits gamma-secretase cleavage of the amyloid precursor protein compared to its effect on amyloid precursor-like protein 1
Abstract
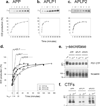
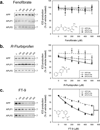
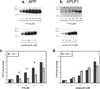
Inhibition of gamma-secretase cleavage of the amyloid precursor protein (APP) is a prime target for the development of therapeutics for treating Alzheimer's disease; however, complete inhibition of this activity would also impair the processing of many other proteins, including the APP homologues, amyloid precursor-like protein (APLP) 1 and 2. To prevent unwanted side effects, therapeutically useful gamma-secretase inhibitors should specifically target APP processing while sparing cleavage of other gamma-substrates. Thus, since APLP1 and APLP2 are more similar to APP than any of the other known gamma-secretase substrates and have important physiological roles in their own right, we reasoned that comparison of the effect of gamma-secretase inhibitors on APLP processing should provide a sensitive indicator of the selectivity of putative inhibitors. To address this issue, we have optimized microsome and cell culture assays to monitor the gamma-secretase proteolysis of APP and APLPs. Production of the gamma-secretase-generated intracellular domain (ICD) occurs more rapidly from APLP1 than from either APLP2 or APP, suggesting that APLP1 is a better gamma-substrate and that substrate recognition is not restricted to the highly conserved amino acid sequences surrounding the epsilon-site. As expected, the well-characterized gamma-secretase modulator, fenofibrate, did not inhibit ICD release, whereas a related compound, FT-9, inhibited gamma-secretase both in microsomes and in whole cells. Importantly, FT-9 displayed a preferential effect, inhibiting cleavage of APP much more effectively than cleavage of APLP1. These findings suggest that selective inhibitors can be developed and that screening of compounds against APP and APLPs should assist in this process.
Figures


Similar articles
-
Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein.Mol Brain. 2008 Nov 4;1:15. doi: 10.1186/1756-6606-1-15. Mol Brain. 2008. PMID: 18983676 Free PMC article.
-
APLP2 is predominantly cleaved by β-secretase and γ-secretase in the human brain.Psychogeriatrics. 2023 Mar;23(2):311-318. doi: 10.1111/psyg.12933. Epub 2023 Jan 23. Psychogeriatrics. 2023. PMID: 36691315
-
gamma-Secretase cleavage and binding to FE65 regulate the nuclear translocation of the intracellular C-terminal domain (ICD) of the APP family of proteins.Biochemistry. 2003 Jun 10;42(22):6664-73. doi: 10.1021/bi027375c. Biochemistry. 2003. PMID: 12779321
-
Proton myo-inositol cotransporter is a novel γ-secretase associated protein that regulates Aβ production without affecting Notch cleavage.FEBS J. 2015 Sep;282(17):3438-51. doi: 10.1111/febs.13353. Epub 2015 Jul 14. FEBS J. 2015. PMID: 26094765 Review.
-
gamma-Secretase as a therapeutic target in Alzheimer's disease.Curr Drug Targets. 2010 Apr;11(4):506-17. doi: 10.2174/138945010790980349. Curr Drug Targets. 2010. PMID: 20015011 Review.
Cited by
-
APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant.EMBO J. 2011 May 17;30(12):2501-9. doi: 10.1038/emboj.2011.161. EMBO J. 2011. PMID: 21587206 Free PMC article.
-
Genomics of Dementia: APOE- and CYP2D6-Related Pharmacogenetics.Int J Alzheimers Dis. 2012;2012:518901. doi: 10.1155/2012/518901. Epub 2012 Mar 14. Int J Alzheimers Dis. 2012. PMID: 22482072 Free PMC article.
-
Amyloid beta 42 peptide (Abeta42)-lowering compounds directly bind to Abeta and interfere with amyloid precursor protein (APP) transmembrane dimerization.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14597-602. doi: 10.1073/pnas.1003026107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679249 Free PMC article.
-
beta-Secretase cleavage is not required for generation of the intracellular C-terminal domain of the amyloid precursor family of proteins.FEBS J. 2010 Mar;277(6):1503-18. doi: 10.1111/j.1742-4658.2010.07579.x. Epub 2010 Feb 15. FEBS J. 2010. PMID: 20163459 Free PMC article.
-
Increased AβPP processing in familial Danish dementia patients.J Alzheimers Dis. 2011;27(2):385-91. doi: 10.3233/JAD-2011-110785. J Alzheimers Dis. 2011. PMID: 21841249 Free PMC article.
References
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. - PubMed
-
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. - PubMed
-
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402:537–540. - PubMed
-
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. - PubMed
-
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that β-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science. 1990;248:492–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

