Photopatterned thiol surfaces for biomolecule immobilization
- PMID: 19821627
- PMCID: PMC2768491
- DOI: 10.1021/la9017135
Photopatterned thiol surfaces for biomolecule immobilization
Abstract
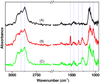
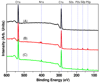
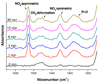
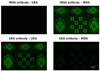
The ability to pattern small molecules and proteins on artificial surfaces is of importance for the development of new tools including tissue engineering, cell-based drug screening, and cell-based sensors. We describe here a novel "caged" thiol-mediated strategy for the fabrication of planar substrates patterned with biomolecules using photolithography. A thiol-bearing phosphoramidite (3-(2'-nitrobenzyl)thiopropyl (NBTP) phosphoramidite) was synthesized and coupled to a hydroxyl-terminated amorphous carbon substrate. A biocompatible oligo(ethylene glycol) spacer was used to resist nonspecific adsorption of protein and DNA and enhance flexibility of attached biomolecules. Thiol functionalities are revealed by UV irradiation of NBTP-modified surfaces. Both the surface coupling and photodeprotection were monitored by Polarization Modulation Fourier Transform Infrared Reflection Absorption Spectroscopy (PM-FTIRRAS) and X-ray Photoelectron Spectroscopy (XPS) measurements. The newly exposed thiols are chemically very active and react readily with a wide variety of groups. A series of molecules including biotin, DNA, and proteins were attached to the surfaces with retention of their biological activities, demonstrating the utility and generality of the approach.
Figures







Similar articles
-
Preparation and photolithography of self-assembled monolayers of 10-mercaptodecanylphosphonic acid on glass mediated by zirconium for protein patterning.Colloids Surf B Biointerfaces. 2013 Aug 1;108:66-71. doi: 10.1016/j.colsurfb.2013.02.030. Epub 2013 Feb 28. Colloids Surf B Biointerfaces. 2013. PMID: 23524079
-
Surface-Anchored Thiol-Reactive Soft Interfaces: Engineering Effective Platforms for Biomolecular Immobilization and Sensing.ACS Appl Mater Interfaces. 2017 Aug 23;9(33):27946-27954. doi: 10.1021/acsami.7b07779. Epub 2017 Aug 8. ACS Appl Mater Interfaces. 2017. PMID: 28745494
-
Influence of amine and thiol modifications at the 3' ends of single stranded DNA molecules on their adsorption on gold surface and the efficiency of their hybridization.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Oct 5;203:31-39. doi: 10.1016/j.saa.2018.05.076. Epub 2018 May 24. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29857258
-
Thiol-based, site-specific and covalent immobilization of biomolecules for single-molecule experiments.Nat Protoc. 2010 Jun;5(6):975-85. doi: 10.1038/nprot.2010.49. Nat Protoc. 2010. PMID: 20448543
-
An OEGylated thiol monolayer for the tethering of liposomes and the study of liposome interactions.Talanta. 2010 Jun 15;81(4-5):1153-61. doi: 10.1016/j.talanta.2010.01.027. Epub 2010 Feb 1. Talanta. 2010. PMID: 20441878
Cited by
-
In situ Synthesis of Oligonucleotide Arrays on Surfaces Coated with Crosslinked Polymer Multilayers.Chem Mater. 2012 Mar 13;24(5):939-945. doi: 10.1021/cm202720q. Epub 2011 Nov 28. Chem Mater. 2012. PMID: 22611305 Free PMC article.
-
Channel surface patterning of alternating biomimetic protein combinations for enhanced microfluidic tumor cell isolation.Anal Chem. 2012 May 1;84(9):4022-8. doi: 10.1021/ac2033408. Epub 2012 Apr 19. Anal Chem. 2012. PMID: 22482510 Free PMC article.
-
Chemistry and material science at the cell surface.Mater Today (Kidlington). 2010 Apr;13(4):14-21. doi: 10.1016/S1369-7021(10)70056-0. Mater Today (Kidlington). 2010. PMID: 21857791 Free PMC article.
-
Carbon Substrates: A Stable Foundation for Biomolecular Arrays.Annu Rev Anal Chem (Palo Alto Calif). 2015;8:263-85. doi: 10.1146/annurev-anchem-071114-040146. Epub 2015 Jun 3. Annu Rev Anal Chem (Palo Alto Calif). 2015. PMID: 26048550 Free PMC article. Review.
-
Quantitative photochemical immobilization of biomolecules on planar and corrugated substrates: a versatile strategy for creating functional biointerfaces.ACS Appl Mater Interfaces. 2011 Sep;3(9):3762-71. doi: 10.1021/am2009597. Epub 2011 Aug 12. ACS Appl Mater Interfaces. 2011. PMID: 21793535 Free PMC article.
References
-
- Khetani SR, Bhatia SN. Curr. Opin. Biotechnol. 2006;17:524–531. - PubMed
-
- Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. FASEB J. 2007;21:790–801. - PubMed
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425–1428. - PubMed
-
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Dev. Cell. 2004;6:483–495. - PubMed
-
- Brockman JM, Frutos AG, Corn RM. J. Am. Chem. Soc. 1999;121:8044–8051.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

