Growth hormone regulates the balance between bone formation and bone marrow adiposity
- PMID: 19821771
- PMCID: PMC3153330
- DOI: 10.1359/jbmr.091015
Growth hormone regulates the balance between bone formation and bone marrow adiposity
Abstract
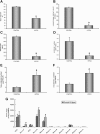
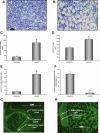
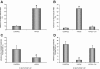
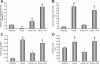
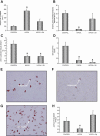
Cancellous bone decreases and bone marrow fat content increases with age. Osteoblasts and adipocytes are derived from a common precursor, and growth hormone (GH), a key hormone in integration of energy metabolism, regulates the differentiation and function of both cell lineages. Since an age-related decline in GH is associated with bone loss, we investigated the relationship between GH and bone marrow adiposity in hypophysectomized (HYPOX) rats and in mice with defects in GH signaling. HYPOX dramatically reduced body weight gain, bone growth and mineralizing perimeter, serum insulin-like growth factor 1 (IGF-1) levels, and mRNA levels for IGF-1 in liver and bone. Despite reduced body mass and adipocyte precursor pool size, HYPOX resulted in a dramatic increase in bone lipid levels, as reflected by increased bone marrow adiposity and bone triglyceride and cholesterol content. GH replacement normalized bone marrow adiposity and precursor pool size, as well as mineralizing perimeter in HYPOX rats. In contrast, 17beta -estradiol, IGF-1, thyroxine, and cortisone were ineffective. Parathyroid hormone (PTH) reversed the inhibitory effects of HYPOX on mineralizing perimeter but had no effect on adiposity. Finally, bone marrow adiposity was increased in mice deficient in GH and IGF-1 but not in mice deficient in serum IGF-1. Taken together, our findings indicate that the reciprocal changes in bone and fat mass in GH signaling-deficient rodents are not directly coupled with one another. Rather, GH enhances adipocyte as well as osteoblast precursor pool size. However, GH increases osteoblast differentiation while suppressing bone marrow lipid accumulation.
Copyright 2010 American Society for Bone and Mineral Research.
Figures
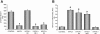

References
-
- Nilsson A, Swolin D, Enerback S, Ohlsson C. Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab. 1995;80:3483–3488. - PubMed
-
- Kasukawa Y, Miyakoshi N, Mohan S. The anabolic effects of GH/IGF system on bone. Curr Pharm Des. 2004;10:2577–2592. - PubMed
-
- Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. - PubMed
-
- Franco C, Bengtsson BA, Johannsson G. The GH/IGF-1 axis in obesity: physiological and pathological aspects. Metab Syndr Relat Disord. 2006;4:51–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

