Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha
- PMID: 19821774
- PMCID: PMC3153331
- DOI: 10.1359/jbmr.091017
Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha
Abstract
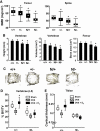
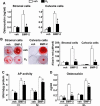
Estrogens diminish oxidative stress in bone and bone marrow, attenuate the generation of osteoblasts, and decrease the prevalence of mature osteoblast apoptosis. We have searched for the molecular mechanism of these effects using as tools a mouse model bearing an estrogen receptor alpha (ERalpha) knock-in mutation that prevents binding to DNA (ERalpha(NERKI/-)) and several osteoblast progenitor cell models expressing the wild-type ERalpha or the ERalpha(NERKI/-). We report that the ability of estrogens to diminish the generation of reactive oxygen species, stimulate the activity of glutathione reductase, and decrease the phosphorylation of p66(shc), as well as osteoblastogenesis and osteoblast number and apoptosis, were fully preserved in ERalpha(NERKI/-) mice, indicating that the DNA-binding function of the ERalpha is dispensable for all these effects. Consistent with the attenuation of osteoblastogenesis in this animal model, 17beta-estradiol attenuated bone morphogenetic protein 2 (BMP-2)-induced gene transcription and osteoblast commitment and differentiation in murine and human osteoblastic cell lines. Moreover, 17beta-estradiol attenuated BMP-2-induced differentiation of primary cultures of calvaria- or bone marrow-derived osteoblastic cells from ERalpha(NERKI/-) mice as effectively as in cells from wild-type littermates. The inhibitory effect of the hormone on BMP-2 signaling resulted from an ERalpha-mediated activation of ERKs and the phosphorylation of Smad1 at the linker region of the protein, which leads to proteasomal degradation. These results illustrate that the effects of estrogens on oxidative stress and the birth and death of osteoblasts do not require the binding of ERalpha to DNA response elements, but instead they result from the activation of cytoplasmic kinases.
Copyright 2010 American Society for Bone and Mineral Research.
Figures





References
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. - PubMed
-
- Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. - PubMed
-
- Manolagas SC, Kousteni S, Chen JR, Schuller M, Plotkin L, Bellido T. Kinase-mediated transcription, activators of nongenotropic estrogen-like signaling (ANGELS), and osteoporosis: a different perspective on the HRT dilemma. Kidney Int Suppl. 2004;91:S41–49. - PubMed
-
- Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

