GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network
- PMID: 19821838
- PMCID: PMC3700401
- DOI: 10.1111/j.1460-9568.2009.06944.x
GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network
Abstract
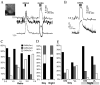
Intercellular communication between gamma-aminobutyric acid (GABA)ergic suprachiasmatic nucleus (SCN) neurons facilitates light-induced phase changes and synchronization of individual neural oscillators within the SCN network. We used ratiometric Ca(2+) imaging techniques to record changes in the intracellular calcium concentration ([Ca(2+)](i)) to study the role of GABA in interneuronal communication and the response of the SCN neuronal network to optic nerve stimulations that mimic entraining light signals. Stimulation of the retinohypothalamic tract (RHT) evoked divergent Ca(2+) responses in neurons that varied regionally within the SCN with a pattern that correlated with those evoked by pharmacological GABA applications. GABA(A) and GABA(B) receptor agonists and antagonists were used to evaluate components of the GABA-induced changes in [Ca(2+)](i). Application of the GABA(A) receptor antagonist gabazine induced changes in baseline [Ca(2+)](i) in a direction opposite to that evoked by GABA, and similarly altered the RHT stimulation-induced Ca(2+) response. GABA application induced Ca(2+) responses varied in time and region within the SCN network. The NKCC1 cotransporter blocker, bumetanide, and L-type calcium channel blocker, nimodipine, attenuated the GABA-induced rise of [Ca(2+)](i). These results suggest that physiological GABA induces opposing effects on [Ca(2+)](i) based on the chloride equilibrium potential, and may play an important role in neuronal Ca(2+) balance, synchronization and modulation of light input signaling in the SCN network.
Figures

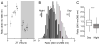
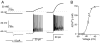








Comment in
-
Excitation by GABA in the SCN reaches its time and place (Commentary on Irwin & Allen).Eur J Neurosci. 2009 Oct;30(8):1461. doi: 10.1111/j.1460-9568.2009.07011.x. Epub 2009 Oct 12. Eur J Neurosci. 2009. PMID: 19821845 No abstract available.
References
-
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. - PubMed
-
- Belenky MA, Sagiv N, Fritschy JM, Yarom Y. Presynaptic and postsynaptic GABAA receptors in rat suprachiasmatic nucleus. Neuroscience. 2003;118:909–923. - PubMed
-
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. The Journal of comparative neurology. 2008;506:708–732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

