A new double-antibody sandwich ELISA targeting Plasmodium falciparum aldolase to evaluate anti-malarial drug sensitivity
- PMID: 19821995
- PMCID: PMC2770540
- DOI: 10.1186/1475-2875-8-226
A new double-antibody sandwich ELISA targeting Plasmodium falciparum aldolase to evaluate anti-malarial drug sensitivity
Abstract
Background: The standard in vitro test to assess anti-malarial activity of chemical compounds is the [3H]hypoxanthine incorporation assay. It is a radioactivity-based method to measure DNA replication of Plasmodium in red blood cells. The method is highly reproducible, however, the handling of radioactive material is costly, hazardous and requires the availability of appropriate technology and trained staff. Several other ways to evaluate in vitro anti-malarial activity do exist, all with their own assets and limitations.
Methods: The newly developed double-antibody sandwich ELISA described here is based on the properties of a non-overlapping pair of monoclonal antibodies directed against Plasmodium falciparum aldolase. This glycolytic enzyme possesses some unique nucleotide sequences compared to the human isoenzymes and has been highly conserved through evolution. Out of twenty possibilities, the most sensitive antibody pair was selected and used to quantitatively detect parasite aldolase in infected blood lysates.
Results: A total of 34 compounds with anti-malarial activity were tested side-by-side by ELISA and the [3H]hypoxanthine incorporation assay. The novel ELISA provided IC 50s closely paralleling those from the radioactivity-based assay (R = 0.99, p < 0.001). At the investigated assay conditions (72 h incubation time, parasitaemia = 0.3%), the assay was found to be reproducible and easy to perform.
Conclusion: The newly developed ELISA presents several advantages over the comparative method, the [3H]hypoxanthine incorporation assay. The assay is highly reproducible, less hazardous (involves no radioactivity) and requires little and cheap technical equipment. Relatively unskilled personnel can conduct this user-friendly assay. All this makes it attractive to be employed in resource-poor laboratories.
Figures
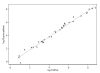
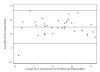
References
-
- Brockman A, Singlam S, Phiaphun L, Looareesuwan S, White NJ, Nosten F. Field evaluation of a novel colorimetric method--double-site enzyme-linked lactate dehydrogenase immunodetection assay--to determine drug susceptibilities of Plasmodium falciparum clinical isolates from northwestern Thailand. Antimicrob Agents Chemother. 2004;48:1426–1429. doi: 10.1128/AAC.48.4.1426-1429.2004. - DOI - PMC - PubMed
-
- Druilhe P, Moreno A, Blanc C, Brasseur PH, Jacquier P. A colorimetric in vitro drug sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site lactate dehydrogenase antigen-capture enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2001;64:233–241. - PubMed
-
- Kaddouri H, Nakache S, Houze S, Mentre F, Le Bras J. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother. 2006;50:3343–3349. doi: 10.1128/AAC.00367-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

