Morphine modulation of pain processing in medial and lateral pain pathways
- PMID: 19822022
- PMCID: PMC2770513
- DOI: 10.1186/1744-8069-5-60
Morphine modulation of pain processing in medial and lateral pain pathways
Abstract
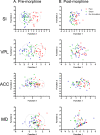
Background: Despite the wide-spread use of morphine and related opioid agonists in clinic and their powerful analgesic effects, our understanding of the neural mechanisms underlying opioid analgesia at supraspinal levels is quite limited. The present study was designed to investigate the modulative effect of morphine on nociceptive processing in the medial and lateral pain pathways using a multiple single-unit recording technique. Pain evoked neuronal activities were simultaneously recorded from the primary somatosensory cortex (SI), ventral posterolateral thalamus (VPL), anterior cingulate cortex (ACC), and medial dorsal thalamus (MD) with eight-wire microelectrode arrays in awake rats.
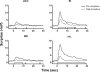
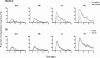
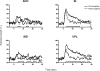
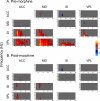
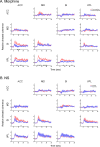
Results: The results showed that the noxious heat evoked responses of single neurons in all of the four areas were depressed after systemic injection of 5 mg/kg morphine. The depressive effects of morphine included (i) decreasing the neuronal response magnitude; (ii) reducing the fraction of responding neurons, and (iii) shortening the response duration. In addition, the capability of cortical and thalamic neural ensembles to discriminate noxious from innocuous stimuli was decreased by morphine within both pain pathways. Meanwhile, morphine suppressed the pain-evoked changes in the information flow from medial to lateral pathway and from cortex to thalamus. These effects were completely blocked by pre-treatment with the opiate receptor antagonist naloxone.
Conclusion: These results suggest that morphine exerts analgesic effects through suppressing both sensory and affective dimensions of pain.
Figures
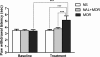


Similar articles
-
Effects of pentobarbital anesthesia on nociceptive processing in the medial and lateral pain pathways in rats.Neurosci Bull. 2010 Jun;26(3):188-96. doi: 10.1007/s12264-010-0150-x. Neurosci Bull. 2010. PMID: 20502496 Free PMC article.
-
The effects of morphine on basal neuronal activities in the lateral and medial pain pathways.Neurosci Lett. 2012 Sep 13;525(2):173-8. doi: 10.1016/j.neulet.2012.07.032. Epub 2012 Jul 24. Neurosci Lett. 2012. PMID: 22841696 Free PMC article.
-
Corticofugal influences on thalamic neurons during nociceptive transmission in awake rats.Synapse. 2007 May;61(5):335-42. doi: 10.1002/syn.20375. Synapse. 2007. PMID: 17318884
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
-
Neuronal nociceptive responses in thalamocortical pathways.Neurosci Bull. 2009 Oct;25(5):289-95. doi: 10.1007/s12264-009-0908-1. Neurosci Bull. 2009. PMID: 19784084 Free PMC article. Review.
Cited by
-
Chronic neuropathic pain in mice reduces μ-opioid receptor-mediated G-protein activity in the thalamus.Brain Res. 2011 Aug 11;1406:1-7. doi: 10.1016/j.brainres.2011.06.023. Epub 2011 Jun 16. Brain Res. 2011. PMID: 21762883 Free PMC article.
-
The neural mobilization technique modulates the expression of endogenous opioids in the periaqueductal gray and improves muscle strength and mobility in rats with neuropathic pain.Behav Brain Funct. 2014 May 13;10:19. doi: 10.1186/1744-9081-10-19. Behav Brain Funct. 2014. PMID: 24884961 Free PMC article.
-
Effects of repeated exposures to experimental cold pain stimulus on pain perception in healthy young Indian men.Med J Armed Forces India. 2022 Sep;78(Suppl 1):S238-S245. doi: 10.1016/j.mjafi.2021.08.002. Epub 2021 Oct 7. Med J Armed Forces India. 2022. PMID: 36147410 Free PMC article.
-
Pain-related cortico-limbic plasticity and opioid signaling.Neuropharmacology. 2023 Jun 15;231:109510. doi: 10.1016/j.neuropharm.2023.109510. Epub 2023 Mar 20. Neuropharmacology. 2023. PMID: 36944393 Free PMC article. Review.
-
Cortico-limbic pain mechanisms.Neurosci Lett. 2019 May 29;702:15-23. doi: 10.1016/j.neulet.2018.11.037. Epub 2018 Nov 29. Neurosci Lett. 2019. PMID: 30503916 Free PMC article. Review.
References
-
- da Fonseca Pacheco D, Klein A, de Castro Perez A, da Fonseca Pacheco CM, de Francischi JN, Duarte ID. The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br J Pharmacol. 2008;154:1143–1149. doi: 10.1038/bjp.2008.175. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

