Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer's and other neurodegenerative diseases
- PMID: 19822029
- PMCID: PMC2874257
- DOI: 10.1186/alzrt5
Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer's and other neurodegenerative diseases
Abstract
Background: Alzheimer's disease (AD) and a host of other neurodegenerative central nervous system (CNS) proteinopathies are characterized by the accumulation of misfolded protein aggregates. Simplistically, these aggregates can be divided into smaller, soluble, oligomeric and larger, less-soluble or insoluble, fibrillar forms. Perhaps the major ongoing debate in the neurodegenerative disease field is whether the smaller oligomeric or larger fibrillar aggregates are the primary neurotoxin. Herein, we propose an integrative hypothesis that provides new insights into how a variety of misfolded protein aggregates can result in neurodegeneration.
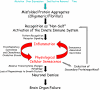
Results: We introduce the concept that a wide range of highly stable misfolded protein aggregates in AD and other neurodegenerative proteinopathies are recognized as non-self and chronically activate the innate immune system. This pro-inflammatory state leads to physiological senescence of CNS cells. Once CNS cells undergo physiological senescence, they secrete a variety of pro-inflammatory molecules. Thus, the senescence of cells, which was initially triggered by inflammatory stimuli, becomes a self-reinforcing stimulus for further inflammation and senescence. Ultimately, senescent CNS cells become functionally impaired and eventually die, and this neurodegeneration leads to brain organ failure.
Conclusion: This integrative hypothesis, which we will refer to as the proteinopathy-induced senescent cell hypothesis of AD and other neurodegenerative diseases, links CNS proteinopathies to inflammation, physiological senescence, cellular dysfunction, and ultimately neurodegeneration. Future studies characterizing the senescent phenotype of CNS cells in AD and other neurodegenerative diseases will test the validity of this hypothesis. The implications of CNS senescence as a contributing factor to the neurodegenerative cascade and its implications for therapy are discussed.
Figures
References
-
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. - PubMed
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–17. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99:689–707. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

