Calcium bridge triggers capsid disassembly in the cell entry process of simian virus 40
- PMID: 19822519
- PMCID: PMC2787333
- DOI: 10.1074/jbc.M109.015107
Calcium bridge triggers capsid disassembly in the cell entry process of simian virus 40
Abstract
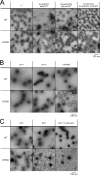
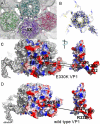
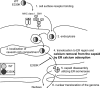
The calcium bridge between the pentamers of polyoma viruses maintains capsid metastability. It has been shown that viral infection is profoundly inhibited by the substitution of lysine for glutamate in one calcium-binding residue of the SV40 capsid protein, VP1. However, it is unclear how the calcium bridge affects SV40 infectivity. In this in vitro study, we analyzed the influence of host cell components on SV40 capsid stability. We used an SV40 mutant capsid (E330K) in which lysine had been substituted for glutamate 330 in protein VP1. The mutant capsid retained the ability to interact with the SV40 cellular receptor GM1, and the internalized mutant capsid accumulated in caveolin-1-mediated endocytic vesicles and was then translocated to the endoplasmic reticulum (ER) region. However, when placed in ER-rich microsome, the mutant capsid retained its spherical structure in contrast to the wild type, which disassembled. Structural analysis of the mutant capsid with cryo-electron microscopy and image reconstruction revealed altered pentamer coordination, possibly as a result of electrostatic interaction, although its overall structure resembled that of the wild type. These results indicate that the calcium ion serves as a trigger at the pentamer interface, which switches on capsid disassembly, and that the failure of the E330K mutant capsid to disassemble is attributable to an inadequate triggering system. Our data also indicate that calcium depletion-induced SV40 capsid disassembly may occur in the ER region and that this is essential for successful SV40 infection.
Figures




Similar articles
-
Interaction between Simian Virus 40 Major Capsid Protein VP1 and Cell Surface Ganglioside GM1 Triggers Vacuole Formation.mBio. 2016 Mar 22;7(2):e00297. doi: 10.1128/mBio.00297-16. mBio. 2016. PMID: 27006465 Free PMC article.
-
Structural basis of GM1 ganglioside recognition by simian virus 40.Proc Natl Acad Sci U S A. 2008 Apr 1;105(13):5219-24. doi: 10.1073/pnas.0710301105. Epub 2008 Mar 19. Proc Natl Acad Sci U S A. 2008. PMID: 18353982 Free PMC article.
-
Importance of calcium-binding site 2 in simian virus 40 infection.J Virol. 2007 Jun;81(11):6099-105. doi: 10.1128/JVI.02195-06. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360742 Free PMC article.
-
Simian virus 40 chromatin interaction with the capsid proteins.J Biomol Struct Dyn. 1983 Dec;1(3):689-704. doi: 10.1080/07391102.1983.10507475. J Biomol Struct Dyn. 1983. PMID: 6101085 Review.
-
Characterization of simian virus 40 on its infectious entry pathway in cells using fluorescence correlation spectroscopy.Biochem Soc Trans. 2004 Nov;32(Pt 5):746-9. doi: 10.1042/BST0320746. Biochem Soc Trans. 2004. PMID: 15494004 Review.
Cited by
-
Principles of Virus Uncoating: Cues and the Snooker Ball.Traffic. 2016 Jun;17(6):569-92. doi: 10.1111/tra.12387. Epub 2016 Mar 31. Traffic. 2016. PMID: 26875443 Free PMC article. Review.
-
Quaternary structures of HIV Env immunogen exhibit conformational vicissitudes and interface diminution elicited by ligand binding.Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):6091-6. doi: 10.1073/pnas.1016113108. Epub 2011 Mar 28. Proc Natl Acad Sci U S A. 2011. PMID: 21444771 Free PMC article.
-
pH stability and disassembly mechanism of wild-type simian virus 40.Soft Matter. 2020 Mar 21;16(11):2803-2814. doi: 10.1039/c9sm02436k. Epub 2020 Feb 27. Soft Matter. 2020. PMID: 32104873 Free PMC article.
-
The structure of avian polyomavirus reveals variably sized capsids, non-conserved inter-capsomere interactions, and a possible location of the minor capsid protein VP4.Virology. 2011 Mar 1;411(1):142-52. doi: 10.1016/j.virol.2010.12.005. Epub 2011 Jan 15. Virology. 2011. PMID: 21239031 Free PMC article.
-
High-resolution x-ray structure and functional analysis of the murine norovirus 1 capsid protein protruding domain.J Virol. 2010 Jun;84(11):5695-705. doi: 10.1128/JVI.00316-10. Epub 2010 Mar 24. J Virol. 2010. PMID: 20335262 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

