A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol
- PMID: 19822655
- PMCID: PMC2798224
- DOI: 10.1128/IAI.00893-09
A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol
Abstract
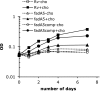
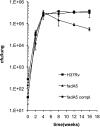
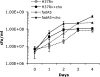
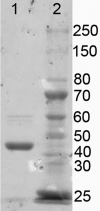
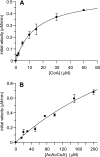
Mycobacterium tuberculosis, the causative agent of tuberculosis, is an intracellular pathogen that shifts to a lipid-based metabolism in the host. Moreover, metabolism of the host lipid cholesterol plays an important role in M. tuberculosis infection. We used transcriptional profiling to identify genes transcriptionally regulated by cholesterol and KstR (Rv3574), a TetR-like repressor. The fadA5 (Rv3546) gene, annotated as a lipid-metabolizing thiolase, the expression of which is upregulated by cholesterol and repressed by KstR, was deleted in M. tuberculosis H37Rv. We demonstrated that fadA5 is required for utilization of cholesterol as a sole carbon source in vitro and for full virulence of M. tuberculosis in the chronic stage of mouse lung infection. Cholesterol is not toxic to the fadA5 mutant strain, and, therefore, toxicity does not account for its attenuation. We show that the wild-type strain, H37Rv, metabolizes cholesterol to androst-4-ene-3,17-dione (AD) and androsta-1,4-diene-3,17-dione (ADD) and exports these metabolites into the medium, whereas the fadA5 mutant strain is defective for this activity. We demonstrate that FadA5 catalyzes the thiolysis of acetoacetyl-coenzyme A (CoA). This catalytic activity is consistent with a beta-ketoacyl-CoA thiolase function in cholesterol beta-oxidation that is required for the production of androsterones. We conclude that the attenuated phenotype of the fadA5 mutant is a consequence of disrupted cholesterol metabolism that is essential only in the persistent stage of M. tuberculosis infection and may be caused by the inability to produce AD/ADD from cholesterol.
Figures


References
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Cardona, P. J., R. Llatjós, S. Gordillo, J. Diaz, I. Ojanguren, A. Aviza, and V. Ausina. 2000. Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis. Scand. J. Immunol. 52:156-163. - PubMed
-
- Chang, J. C., N. S. Harik, R. P. Liao, and D. R. Sherman. 2007. Identification of mycobacterial genes that alter growth and pathology in macrophages and in mice. J. Infect. Dis. 196:788-795. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

