Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock
- PMID: 19822660
- PMCID: PMC2786870
- DOI: 10.1128/MCB.00337-09
Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock
Abstract
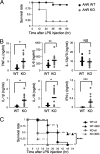
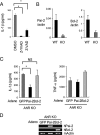
Aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, is known to mediate a wide variety of pharmacological and toxicological effects caused by polycyclic aromatic hydrocarbons. Recent studies have revealed that AhR is involved in the normal development and homeostasis of many organs. Here, we demonstrate that AhR knockout (AhR KO) mice are hypersensitive to lipopolysaccharide (LPS)-induced septic shock, mainly due to the dysfunction of their macrophages. In response to LPS, bone marrow-derived macrophages (BMDM) of AhR KO mice secreted an enhanced amount of interleukin-1beta (IL-1beta). Since the enhanced IL-1beta secretion was suppressed by supplementing Plasminogen activator inhibitor-2 (Pai-2) expression through transduction with Pai-2-expressing adenoviruses, reduced Pai-2 expression could be a cause of the increased IL-1beta secretion by AhR KO mouse BMDM. Analysis of gene expression revealed that AhR directly regulates the expression of Pai-2 through a mechanism involving NF-kappaB but not AhR nuclear translocator (Arnt), in an LPS-dependent manner. Together with the result that administration of the AhR ligand 3-methylcholanthrene partially protected mice with wild-type AhR from endotoxin-induced death, these results raise the possibility that an appropriate AhR ligand may be useful for treating patients with inflammatory disorders.
Figures




Comment in
-
LeA(H)Rning self-control.Cell Res. 2014 Oct;24(10):1155-6. doi: 10.1038/cr.2014.96. Epub 2014 Jul 25. Cell Res. 2014. PMID: 25060701 Free PMC article.
References
-
- Abbott, B., J. Schmid, J. Pitt, A. Buckalew, C. Wood, G. Held, and J. Diliberto. 1999. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol. Appl. Pharmacol. 155:62-70. - PubMed
-
- Blasi, E., D. Radzioch, S. Durum, and L. Varesio. 1987. A murine macrophage cell line, immortalized by v-raf and v-myc oncogenes, exhibits normal macrophage functions. Eur. J. Immunol. 17:1491-1498. - PubMed
-
- Bruey, J., N. Bruey-Sedano, F. Luciano, D. Zhai, R. Balpai, C. Xu, C. Kress, B. Bailly-Maitre, X. Li, A. Osterman, S. Matsuzawa, A. Terskikh, B. Faustin, and J. Reed. 2007. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129:45-56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
