Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals
- PMID: 19822663
- PMCID: PMC2786873
- DOI: 10.1128/MCB.00489-09
Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals
Abstract
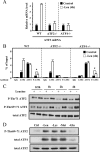
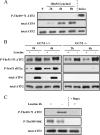
It has been well established that amino acid availability can control gene expression. Previous studies have shown that amino acid depletion induces transcription of the ATF3 (activation transcription factor 3) gene through an amino acid response element (AARE) located in its promoter. This event requires phosphorylation of activating transcription factor 2 (ATF2), a constitutive AARE-bound factor. To identify the signaling cascade leading to phosphorylation of ATF2 in response to amino acid starvation, we used an individual gene knockdown approach by small interfering RNA transfection. We identified the mitogen-activated protein kinase (MAPK) module MEKK1/MKK7/JNK2 as the pathway responsible for ATF2 phosphorylation on the threonine 69 (Thr69) and Thr71 residues. Then, we progressed backwards up the signal transduction pathway and showed that the GTPase Rac1/Cdc42 and the protein Galpha12 control the MAPK module, ATF2 phosphorylation, and AARE-dependent transcription. Taken together, our data reveal a new signaling pathway activated by amino acid starvation leading to ATF2 phosphorylation and subsequently positively affecting the transcription of amino acid-regulated genes.
Figures







References
-
- Averous, J., A. Bruhat, C. Jousse, V. Carraro, G. Thiel, and P. Fafournoux. 2004. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J. Biol. Chem. 279:5288-5297. - PubMed
-
- Baan, B., H. van Dam, G. C. van der Zon, J. A. Maassen, and D. M. Ouwens. 2006. The role of c-Jun N-terminal kinase, p38, and extracellular signal-regulated kinase in insulin-induced Thr69 and Thr71 phosphorylation of activating transcription factor 2. Mol. Endocrinol. 20:1786-1795. - PubMed
-
- Baertl, J. M., R. P. Placko, and G. G. Graham. 1974. Serum proteins and plasma free amino acids in severe malnutrition. Am. J. Clin. Nutr. 27:733-742. - PubMed
-
- Barbosa-Tessmann, I. P., C. Chen, C. Zhong, F. Siu, S. M. Schuster, H. S. Nick, and M. S. Kilberg. 2000. Activation of the human asparagine synthetase gene by the amino acid response and the endoplasmic reticulum stress response pathways occurs by common genomic elements. J. Biol. Chem. 275:26976-26985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
