Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains
- PMID: 19822673
- PMCID: PMC2768828
- DOI: 10.1083/jcb.200903098
Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains
Abstract
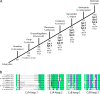
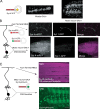
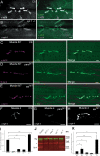
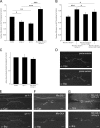
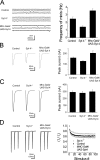
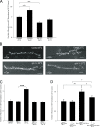
Ca(2+) influx into synaptic compartments during activity is a key mediator of neuronal plasticity. Although the role of presynaptic Ca(2+) in triggering vesicle fusion though the Ca(2+) sensor synaptotagmin 1 (Syt 1) is established, molecular mechanisms that underlie responses to postsynaptic Ca(2+) influx remain unclear. In this study, we demonstrate that fusion-competent Syt 4 vesicles localize postsynaptically at both neuromuscular junctions (NMJs) and central nervous system synapses in Drosophila melanogaster. Syt 4 messenger RNA and protein expression are strongly regulated by neuronal activity, whereas altered levels of postsynaptic Syt 4 modify synaptic growth and presynaptic release properties. Syt 4 is required for known forms of activity-dependent structural plasticity at NMJs. Synaptic proliferation and retrograde signaling mediated by Syt 4 requires functional C2A and C2B Ca(2+)-binding sites, as well as serine 284, an evolutionarily conserved substitution for a key Ca(2+)-binding aspartic acid found in other synaptotagmins. These data suggest that Syt 4 regulates activity-dependent release of postsynaptic retrograde signals that promote synaptic plasticity, similar to the role of Syt 1 as a Ca(2+) sensor for presynaptic vesicle fusion.
Figures


References
-
- Barber C.F., Littleton J.T.. 2007. Synaptic growth and transcriptional regulation in Drosophila. In Transcriptional Regulation by Neuronal Activity: To the Nucleus and Back. Dudek S., editor. Springer Science Publishing, New York. 253–275.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

