Solution structure of a paradigm ArsR family zinc sensor in the DNA-bound state
- PMID: 19822742
- PMCID: PMC2775347
- DOI: 10.1073/pnas.0905558106
Solution structure of a paradigm ArsR family zinc sensor in the DNA-bound state
Abstract
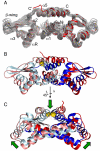
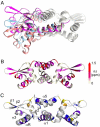
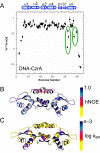
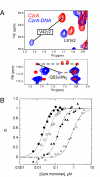
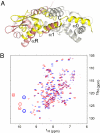
Staphylococcus aureus CzrA is a zinc-dependent transcriptional repressor from the ubiquitous ArsR family of metal sensor proteins. Zn(II) binds to a pair of intersubunit C-terminal alpha5-sensing sites, some 15 A distant from the DNA-binding interface, and allosterically inhibits DNA binding. This regulation is characterized by a large allosteric coupling free energy (DeltaGc) of approximately +6 kcal mol(-1), the molecular origin of which is poorly understood. Here, we report the solution quaternary structure of homodimeric CzrA bound to a palindromic 28-bp czr operator, a structure that provides an opportunity to compare the two allosteric "end" states of an ArsR family sensor. Zn(II) binding drives a quaternary structural switch from a "closed" DNA-binding state to a low affinity "open" conformation as a result of a dramatic change in the relative orientations of the winged helical DNA binding domains within the dimer. Zn(II) binding also effectively quenches both rapid and intermediate timescale internal motions of apo-CzrA while stabilizing the native state ensemble. In contrast, DNA binding significantly enhances protein motions in the allosteric sites and reduces the stability of the alpha5 helices as measured by H-D solvent exchange. This study reveals how changes in the global structure and dynamics drive a long-range allosteric response in a large subfamily of bacterial metal sensor proteins, and provides insights on how other structural classes of ArsR sensor proteins may be regulated by metal binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. - PubMed
-
- Ma Z, Jacobsen FE, Giedroc DP. Metal transporters and metal sensors: How coordination chemistry controls bacterial metal homeostasis. Chem Rev. 2009;109 10.1021/cr900077w. - DOI - PMC - PubMed
-
- Giedroc DP, Arunkumar AI. Metal sensor proteins: Nature's metalloregulated allosteric switches. Dalton Trans. 2007:3107–3120. - PubMed
-
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

