Glutathione S-transferase-micro1 regulates vascular smooth muscle cell proliferation, migration, and oxidative stress
- PMID: 19822795
- PMCID: PMC2783903
- DOI: 10.1161/HYPERTENSIONAHA.109.139428
Glutathione S-transferase-micro1 regulates vascular smooth muscle cell proliferation, migration, and oxidative stress
Abstract
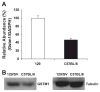
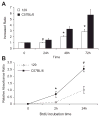
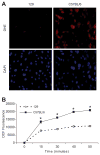
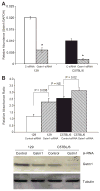
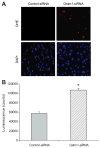
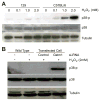
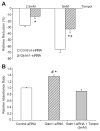
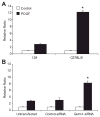
Glutathione S-transferase-micro1, GSTM1, belongs to a superfamily of glutathione S-transferases that metabolizes a broad range of reactive oxygen species and xenobiotics. Across species, genetic variants that result in decreased expression of the Gstm1 gene are associated with increased susceptibility for vascular diseases, including atherosclerosis in humans. We previously identified Gstm1 as a positional candidate in our gene mapping study for susceptibility to renal vascular injury characterized by medial hypertrophy and hyperplasia of the renal vessels. To determine the role of Gstm1 in vascular smooth muscle cells (VSMCs), we isolated VSMCs from mouse aortas. We demonstrate that VSMCs from the susceptible C57BL/6 mice have reduced expression of Gstm1 mRNA and its protein product compared with that of the resistant 129 mice. After serum stimulation, C57BL/6 VSMCs proliferate and migrate at a much faster rate than 129 VSMCs. Furthermore, C57BL/6 VSMCs have higher levels of reactive oxygen species and exhibit exaggerated p38 mitogen-activated protein kinase phosphorylation after exposure to H(2)O(2). To establish causality, we show that knockdown of Gstm1 by small interfering RNA results in increased proliferation of VSMCs in a dose-dependent manner, as well as in increased reactive oxygen species levels and VSMC migration. Moreover, Gstm1 small interfering RNA causes increased p38 mitogen-activated protein kinase phosphorylation and attenuates the antiproliferative effect of Tempol. Our data suggest that Gstm1 is a novel regulator of VSMC proliferation and migration through its role in handling reactive oxygen species. Genetic variants that cause a decremental change in expression of Gstm1 may permit an environment of exaggerated oxidative stress, leading to susceptibility to vascular remodeling and atherosclerosis.
Figures
References
-
- Landi S, Norppa H, Frenzilli G, Cipollini G, Ponzanelli I, Barale R, Hirvonen A. Individual sensitivity to cytogenetic effects of 1,2:3,4-diepoxybutane in cultured human lymphocytes: influence of glutathione S-transferase M1, P1 and T1 genotypes. Pharmacogenetics. 1998;8:461–471. - PubMed
-
- Strange RC, Faulder CG, Davis BA, Hume R, Brown JA, Cotton W, Hopkinson DA. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984;48:11–20. - PubMed
-
- Takahashi Y, Campbell EA, Hirata Y, Takayama T, Listowsky I. A basis for differentiating among the multiple human Mu-glutathione S-transferases and molecular cloning of brain GSTM5. J Biol Chem. 1993;268:8893–8898. - PubMed
-
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

