Plasmid-mediated quinolone resistance: a multifaceted threat
- PMID: 19822894
- PMCID: PMC2772364
- DOI: 10.1128/CMR.00016-09
Plasmid-mediated quinolone resistance: a multifaceted threat
Abstract
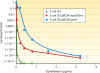
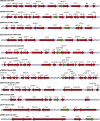
Although plasmid-mediated quinolone resistance (PMQR) was thought not to exist before its discovery in 1998, the past decade has seen an explosion of research characterizing this phenomenon. The best-described form of PMQR is determined by the qnr group of genes. These genes, likely originating in aquatic organisms, code for pentapeptide repeat proteins. These proteins reduce susceptibility to quinolones by protecting the complex of DNA and DNA gyrase or topoisomerase IV enzymes from the inhibitory effect of quinolones. Two additional PMQR mechanisms were recently described. aac(6')-Ib-cr encodes a variant aminoglycoside acetyltransferase with two amino acid alterations allowing it to inactivate ciprofloxacin through the acetylation of its piperazinyl substituent. oqxAB and qepA encode efflux pumps that extrude quinolones. All of these genes determine relatively small increases in the MICs of quinolones, but these changes are sufficient to facilitate the selection of mutants with higher levels of resistance. The contribution of these genes to the emergence of quinolone resistance is being actively investigated. Several factors suggest their importance in this process, including their increasing ubiquity, their association with other resistance elements, and their emergence simultaneous with the expansion of clinical quinolone resistance. Of concern, these genes are not yet being taken into account in resistance screening by clinical microbiology laboratories.
Figures

References
-
- Adams-Haduch, J. M., D. L. Paterson, H. E. Sidjabat, A. W. Pasculle, B. A. Potoski, C. A. Muto, L. H. Harrison, and Y. Doi. 2008. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob. Agents Chemother. 52:3837-3843. - PMC - PubMed
-
- Adjei, M. D., T. M. Heinze, J. Deck, J. P. Freeman, A. J. Williams, and J. B. Sutherland. 2007. Acetylation and nitrosation of ciprofloxacin by environmental strains of mycobacteria. Can. J. Microbiol. 53:144-147. - PubMed
-
- Al-Ahmad, A., F. D. Daschner, and K. Kummerer. 1999. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch. Environ. Contam. Toxicol. 37:158-163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

