Inhibition of RNA recruitment and replication of an RNA virus by acridine derivatives with known anti-prion activities
- PMID: 19823675
- PMCID: PMC2757906
- DOI: 10.1371/journal.pone.0007376
Inhibition of RNA recruitment and replication of an RNA virus by acridine derivatives with known anti-prion activities
Abstract
Background: Small molecule inhibitors of RNA virus replication are potent antiviral drugs and useful to dissect selected steps in the replication process. To identify antiviral compounds against Tomato bushy stunt virus (TBSV), a model positive stranded RNA virus, we tested acridine derivatives, such as chlorpromazine (CPZ) and quinacrine (QC), which are active against prion-based diseases.
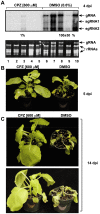
Methodology/principal findings: Here, we report that CPZ and QC compounds inhibited TBSV RNA accumulation in plants and in protoplasts. In vitro assays revealed that the inhibitory effects of these compounds were manifested at different steps of TBSV replication. QC was shown to have an effect on multiple steps, including: (i) inhibition of the selective binding of the p33 replication protein to the viral RNA template, which is required for recruitment of viral RNA for replication; (ii) reduction of minus-strand synthesis by the tombusvirus replicase; and (iii) inhibition of translation of the uncapped TBSV genomic RNA. In contrast, CPZ was shown to inhibit the in vitro assembly of the TBSV replicase, likely due to binding of CPZ to intracellular membranes, which are important for RNA virus replication.
Conclusion/significance: Since we found that CPZ was also an effective inhibitor of other plant viruses, including Tobacco mosaic virus and Turnip crinkle virus, it seems likely that CPZ has a broad range of antiviral activity. Thus, these inhibitors constitute effective tools to study similarities in replication strategies of various RNA viruses.
Conflict of interest statement
Figures






Similar articles
-
Identification of small molecule inhibitors of Tomato bushy stunt virus replication.Methods Mol Biol. 2012;894:345-57. doi: 10.1007/978-1-61779-882-5_23. Methods Mol Biol. 2012. PMID: 22678591
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
Co-opted Cellular Sac1 Lipid Phosphatase and PI(4)P Phosphoinositide Are Key Host Factors during the Biogenesis of the Tombusvirus Replication Compartment.J Virol. 2020 Jun 1;94(12):e01979-19. doi: 10.1128/JVI.01979-19. Print 2020 Jun 1. J Virol. 2020. PMID: 32269127 Free PMC article.
-
Tombusvirus-Host Interactions: Co-Opted Evolutionarily Conserved Host Factors Take Center Court.Annu Rev Virol. 2016 Sep 29;3(1):491-515. doi: 10.1146/annurev-virology-110615-042312. Epub 2016 Aug 8. Annu Rev Virol. 2016. PMID: 27578441 Review.
-
Global genomics and proteomics approaches to identify host factors as targets to induce resistance against Tomato bushy stunt virus.Adv Virus Res. 2010;76:123-77. doi: 10.1016/S0065-3527(10)76004-8. Epub 2010 Mar 31. Adv Virus Res. 2010. PMID: 20965073 Free PMC article. Review.
Cited by
-
The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses.Planta. 2011 Jul;234(1):171-81. doi: 10.1007/s00425-011-1391-2. Epub 2011 Mar 11. Planta. 2011. PMID: 21394469
-
Natural Compounds as Non-Nucleoside Inhibitors of Zika Virus Polymerase through Integration of In Silico and In Vitro Approaches.Pharmaceuticals (Basel). 2022 Nov 30;15(12):1493. doi: 10.3390/ph15121493. Pharmaceuticals (Basel). 2022. PMID: 36558945 Free PMC article.
-
Inhibition of encephalomyocarditis virus and poliovirus replication by quinacrine: implications for the design and discovery of novel antiviral drugs.J Virol. 2010 Sep;84(18):9390-7. doi: 10.1128/JVI.02569-09. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631142 Free PMC article.
-
Antiviral activity of an N-allyl acridone against dengue virus.J Biomed Sci. 2015 Apr 17;22(1):29. doi: 10.1186/s12929-015-0134-2. J Biomed Sci. 2015. PMID: 25908170 Free PMC article.
-
The GEF1 proton-chloride exchanger affects tombusvirus replication via regulation of copper metabolism in yeast.J Virol. 2013 Feb;87(3):1800-10. doi: 10.1128/JVI.02003-12. Epub 2012 Nov 28. J Virol. 2013. PMID: 23192874 Free PMC article.
References
-
- Thompson A, Patel K, Tillman H, McHutchison JG. Directly acting antivirals for the treatment of patients with hepatitis C infection: a clinical development update addressing key future challenges. J Hepatol. 2009;50:184–194. - PubMed
-
- Toniutto P, Fabris C, Bitetto D, Fumolo E, Fornasiere E, et al. R-1626, a specific oral NS5B polymerase inhibitor of hepatitis C virus. IDrugs. 2008;11:738–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

