LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue
- PMID: 19823686
- PMCID: PMC2758584
- DOI: 10.1371/journal.pone.0007422
LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue
Abstract
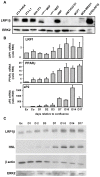
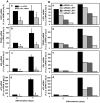
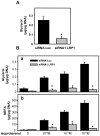
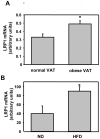
The cell surface low-density lipoprotein receptor-related protein 1, LRP1, plays a major role in lipid metabolism. The question that remains open concerns the function of LRP1 in adipogenesis. Here, we show that LRP1 is highly expressed in murine preadipocytes as well as in primary culture of human adipocytes. Moreover, LRP1 remains abundantly synthesised during mouse and human adipocyte differentiation. We demonstrate that LRP1 silencing in 3T3F442A murine preadipocytes significantly inhibits the expression of PPARgamma, HSL and aP2 adipocyte differentiation markers after adipogenesis induction, and leads to lipid-depleted cells. We further show that the absence of lipids in LRP1-silenced preadipocytes is not caused by lipolysis induction. In addition, we provide the first evidences that LRP1 is significantly up-regulated in obese C57BI6/J mouse adipocytes and obese human adipose tissues. Interestingly, silencing of LRP1 in fully-differentiated adipocytes also reduces cellular lipid level and is associated with an increase of basal lipolysis. However, the ability of mature adipocytes to induce lipolysis is independent of LRP1 expression. Altogether, our findings highlight the dual role of LRP1 in the control of adipogenesis and lipid homeostasis, and suggest that LRP1 may be an important therapeutic target in obesity.
Conflict of interest statement
Figures





References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. - PubMed
-
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8(10):1224–1234. - PubMed
-
- Gauthier A, Vassiliou G, Benoist F, McPherson R. Adipocyte low density lipoprotein receptor-related protein gene expression and function is regulated by peroxisome proliferator-activated receptor gamma. J Biol Chem. 2003;278(14):11945–11953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

