Key factors in mTOR regulation
- PMID: 19823764
- PMCID: PMC4780839
- DOI: 10.1007/s00018-009-0163-7
Key factors in mTOR regulation
Abstract
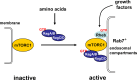
Mammalian target of rapamycin (mTOR) is a protein serine/threonine kinase that controls a wide range of growth-related cellular processes. In the past several years, many factors have been identified that are involved in controlling mTOR activity. Those factors in turn are regulated by diverse signaling cascades responsive to changes in intracellular and environmental conditions. The molecular connections between mTOR and its regulators form a complex signaling network that governs cellular metabolism, growth and proliferation. In this review, we discuss some key factors in mTOR regulation and mechanisms by which these factors control mTOR activity.
Figures




References
-
- De Virgilio C, Loewith R. Cell growth control: little eukaryotes make big contributions. Oncogene. 2006;25:6392–6415. - PubMed
-
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

